Translate this page into:
Primary Systemic Therapy for HER2/Neu-Positive Operable Breast Cancer Increases the Number of Breast-Conserving Surgery and Disease-Free Survival: Retrospective Cohort Analysis at Single Institution
Address for correspondence Yohana Azhar, MD, PhD, Department of Surgery, Division Oncology Head and Neck Surgery, Hasan Sadikin General Hospital/Faculty of Medicine, Universitas Padjadjaran, Pasteur No.38, Bandung, West Java 40161, Indonesia. yohanaspbonk@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective The aim of this study was to evaluate the efficacy and cardiotoxicity profile, and to reduce the extend of breast cancer surgery in primary systemic therapy (PST) HER2/neu–positive operable breast cancer patients.
Materials and Methods A total of 152 patients diagnosed from 2010 to 2015 were included in the study. The PST consisted of a sequential regimen of taxanes and anthracyclines plus trastuzumab. The clinical and pathological responses and the type of breast cancer surgery were evaluated and correlated with clinical and biological factors. The cardiotoxicity profile and long-term benefits were analyzed.
Results The median patient age was 47 (37–67) years, with T2 and T3 67 (44.1%) and 85 (55.9%), respectively. Axillary lymph node breast cancer at diagnosis N0 was 104 (68.4%) and N1 and N2 were 28.9% and 2.6%, respectively. A total of 95.7% of patients had nonspecific type of breast cancer, 67% of tumors were hormonal receptor–negative, 75.5% were grade III, 100% Ki67 > 20%, and 90% of tumors were confirmed to be HER2/neu–positive through immunohistochemistry. Following PST, pathological complete response (pCR) rate was achieved in 44.7% evaluable patients. The pCR rate was higher in HR-negative (93.1% vs. 6.9%) cancer and in grade III (86.2%) than in grade I and II (13.8%) cancer; only 75.5% of complete response (CR) on ultrasound and magnetic resonance imaging were also CR on pathology results. Breast conserving surgery was performed in 41.4%. Regarding type of chemotherapy, there were no significant differences between chemotherapy with anthracycline backbone or taxanes to achieved pathological complete response. Despite that, we were unable to demonstrate an association between pCR and better DFS with p = 0.096; HR 5.7 95.0% CI (0.73–45.52). Patients who are hormonal receptor positive tend to have lower disease-free survival (DFS) than those who are hormonal receptor negative; HR = 6.34, 95.0% CI (1.54–26.00) and p = 0.010. Five years DFS was higher for those who achieved pCR compare with those who did not. Even in this research we failed to show it is statistically significant.
Conclusion A sequential regimen of taxanes and anthracyclines plus trastuzumab was effective with high pCR rates and increases the possibility to do breast conservation surgery and had tolerable cardiotoxicity profile.
Keywords
primary systemic therapy
HER2/neu operable breast cancer
breast conserving surgery
pathological complete response
disease-free survival
Introduction
Previously primary systemic therapy (PST) was used as standard therapy for locally advanced breast cancer. In this setting, PST was given to achieve safety margin and the objective response rates were high with a substantial proportion of patients manageable to surgery. At the same time PST was also given to the patients who willing to preserve the breast, but at that time were not a candidate for breast conservation surgery (BCS).1,2 Many investigators have demonstrated that the rate of BCS is significantly higher in patients treated with PST compared with those who received adjuvant chemotherapy; moreover the risk of ipsilateral recurrence remains no different, while survival is at least as good as with adjuvant systemic therapy.3
Recently there is a growing evidence that PST not only has benefit for allowing BCS but can also be used for predicting long-term outcome in high-risk breast cancer subtype, to give early access for highly effective drugs, and to provide an incentive drug development in breast cancer subtypes with unmet need. Some controversial issues arose in developing countries. In Indonesia as one of the developing countries, this new guideline was hardly acceptable due to some uncertain evidence between relationship of pathological complete response (pCR) and long-term outcome, balancing between efficacy that outweighs exposure of curable patients to agents that may increase toxicity and financial burden.2,3,4,5,6,7,8
We analyze the efficacy, potential to reduce the extend of surgery, toxicity, and long-term follow-up in 152 patients with Her2-positive breast cancer tumors, diagnosed from 2010 to 2015 at a single institution in Bandung, West Java, Indonesia, who were treated with PST in HER2/neu-positive operable breast cancer. In the future, this data might be used as a consideration to change the protocol of our institution regarding the high-risk operable breast cancer management in our institution.
Materials and Methods
This is a retrospective observational study that included 152 patients with stage I and II of HER2/neu-positive breast cancer diagnosed from 2010 to 2015 at Hasan Sadikin General Hospital, Bandung, West Java, Indonesia. The study was approved by the ethics committee of the Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, West Java, Indonesia.
We retrospectively reviewed a series of 152 patients with operable HER2/neu breast cancer who met the criteria mentioned earlier from the medical records of Hasan Sadikin General Hospital. The PST regimens included anthracycline backbone and taxane backbone containing transtuzumab regimen. All patients were 18 years of age or older and had Karnoffsky score of ≥90. Histological type, tumor grade, Ki-67 index, estrogen and progesterone receptor, and HER2 status were determined locally using pretreatment core biopsies. HER2-positive breast cancer was assessed using immunohistochemical techniques or using fluorescent in situ hybridization (FISH) or double in situ hybridization (DISH). An ultrasound-guided fine-needle puncture aspiration was also performed on suspected malignant axillary lymph nodes at diagnosis. The tumor site was marked.
Sentinel lymph node biopsy (SLNB) was performed in patients with clinically negative axilla and for patients who were previously positive. We did axillary dissection even though after PST the patient converted to be clinically negative.9,10 Patients were assessed and it was ensured that they do not have cardiovascular disease and demonstrated adequate cardiac functions with measure left ventricular ejection fraction (LVEF) above 50, using echocardiography. This echocardiography was repeated after three cycles, at the end of chemotherapy and during the follow-up period, at least 6 months after the end of adjuvant trastuzumab. Patients were also assessed for hematological, renal, and hepatic dysfunction. The different treatment regimens administered are shown in (Table 1). The majority of patients received a total of eight cycles consisting of a sequence of anthracyclines and taxanes with trastuzumab.
|
Variable |
Total (n = 152), Percentage (%) |
Pathological complete response (CR) |
p-Value |
|
|---|---|---|---|---|
|
Positive |
Negative |
|||
|
Age (years old) |
0.630a |
|||
|
Mean ± std |
47.3 ±5.7 |
47.0 ± 5.2 |
47.8 ± 6.6 |
|
|
Median |
46.50 (37.00–67.00) |
46.00 (37.00–60.00) |
47.00 (37.00–67.00) |
|
|
Histopathology |
NS |
|||
|
Lobular |
1 (0.7%) |
1 (1.1%) |
0 (0.0%) |
|
|
Medullary |
2 (1.3%) |
2 (2.1%) |
0 (0.0%) |
|
|
Mixed type |
1 (0.7%) |
0 (0.0%) |
1(1.7%) |
|
|
Mucinous |
1 (0.7%) |
0 (0.0%) |
1 (1.7%) |
|
|
IDC-NOS |
146 (96.1%) |
90 (95.7%) |
56 (96.6%) |
|
|
Signet carcinoma |
1 (0.7%) |
1(1.1%) |
0 (0.0%) |
|
|
Tumor size |
67 (44.1%) |
42 (44.7%) |
25 (43.1%) |
0.849 |
|
T2 |
85 (55.9%) |
52 (55.3%) |
33 (56.9%) |
|
|
T3 |
||||
|
Axillary lymph node |
0.844 |
|||
|
N0 |
104 (68.4%) |
36 (62.1%) |
68 (72.3%) |
|
|
N1 |
44 (28.9%) |
23 (24.5%) |
21 (36.2%) |
|
|
N2 |
4 (2.6%) |
3 (3.2%) |
1 (1.7%) |
|
|
Grade |
0.113 |
|||
|
II |
31 (20.4%) |
23 (24.5%) |
8 (13.8%) |
|
|
III |
121 (79.6%) |
71 (75.5%) |
50 (86.2%) |
|
|
Hormonal Status |
< 0.05b |
|||
|
Positive |
35 (23.0%) |
31 (33.0%) |
4 (6.9%) |
|
|
Negative |
117 (77.0%) |
63 (67.0%) |
54 (93.1%) |
|
|
Ki67 |
NS |
|||
|
< 20% |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
|
> 20% |
152 (100.0%) |
72 (47.4%) |
80 (52.6%) |
|
|
Type of chemotherapy |
||||
|
AC-TH |
101 (66.4%) |
64 (68.1%) |
37 (63.8%) |
0.998 |
|
EC-TH |
8 (5.3%) |
5 (5.3%) |
3 (5.2%) |
|
|
FEC |
2 (1.3%) |
2 (2.1%) |
0 (0.0%) |
|
|
TC |
41 (27.0%) |
23 (24.5%) |
18 (31.0%) |
|
Abbreviations: IDC-NOS, invasive ductal carcinoma—no special type; N0, no regional lymph node metastases; N1, metastases (by imaging or clinical examination); N1, metastases to movable ipsilateral level I, II axillary lymph node; N2, metastases in ipsilateral level I, II, axillary lymph nodes that are clinically fixed or matte; N3, ipsilateral internal mammary node involvement; T,N,M (based on American Joint Committee on Cancer 7th edition); T1, tumor ≤ 2 cm; T2, tumor > 20 mm but < 50 mm in greatest dimension; T3, tumor > 50 mm in greatest dimension; T4, tumor of any size with direct extension to the chest wall and/or to the skin.
at-test
bSignificant using chi-square test
Physical examinations were performed every 3 weeks during chemotherapy treatment. Mammograms, ultrasound, or magnetic resonance imaging (MRI) were performed before and after neoadjuvant treatment. The clinical response was determined through physical examination and imaging in accordance with the response evaluation criteria in solid tumors.
Patients underwent surgery between 3 and 5 weeks from the end of chemotherapy. Pathological complete response (pCR) was defined as the total absence of invasive tumor in both breasts and axillary nodes (ypT0/is ypN0). The pathological response was measured using Miller and Payne score. We used the revised American Joint Committee on Cancer TNM system in patients included in the first 2 years of the study. This pathological response was correlated with clinical and biological factors (tumor size, axillary nodes, hormonal status) and Ki-67 index.
All patients who underwent breast-conserving surgery also received whole-breast irradiation at a standard dose. Regional nodal irradiation of the supraclavicular and fossa-axillary were done for patients with four or more positive lymph nodes after giving primary systemic chemotherapy. After completion of systemic and local therapy, patients with hormonal receptor–positive tumors were given hormonal therapy.
Statistical Analysis
We performed a descriptive analysis for all variables. Continuous variables were reported using the median and the standard deviation (SD). For dichotomous or categorical variables, absolute numbers and percentages were computed. The chi-squared two-tailed test was used for comparative analysis between categorical variables.
We estimated disease-free survival (DFS) rates for each group using the Kaplan–Meier method. A comparison of survival curves was performed using the log-rank test. Differences in survival between groups were compared using the Cox regression test. All statistical tests were two sided, and a significance level of 0.05 was applied.
Results
A total of 152 patients with stage I and II HER2/neu-positive breast cancer tumors, who were candidates for primary systemic therapy, were diagnosed from 2010 to 2014 at our institution. The regimen types of the patients are listed at medical record including docetaxel, 75 mg/m2 every 3 weeks; FEC (5-fluorouracil), 600 mg/m2; epirubicin, 75 mg/m2; and cyclophosphamide, 600 mg/m2 every 3 weeks at four cycles. Transtuzumab 8 mg/kg intravenous (IV) loading dose was administered, followed by 6 mg/kg IV every 3 weeks in a year, including in the neoadjuvant and adjuvant setting.
The median patient age was 47 (37–67) years, with T2 and T3 67 (44.1%) and 85 (55.9%), respectively. Axillary lymph node breast cancer at diagnosis N0 was 104 (68.4%) and N1 and N2 were 28.9% and 2.6%, respectively. A total of 95.7% patients had nonspecific type of breast cancer, 67% of tumors were hormonal receptor–negative, 75.5% were grade III, and 100% Ki67 > 20%, while 90% of tumors were confirmed to be HER2/neu-positive through immunohistochemistry (3-fold increase in protein expression) and 10% through FISH or DISH examination. A total of 94 (7%) of patients had an indication for mastectomy at diagnosis. All the patients completed the planned cycles of chemotherapy (Table 1).
Clinical Response
The clinical complete response (cCR) was assessed through mammography, ultrasound imaging, and MRI before and after systemic therapy and immediately before surgery in most patients. In the 2 years of the study, breast MRI was not a routine imaging technique; so, it was not performed in the first 38 patients (Table 2).
|
Imaging breast |
Total patients (n) |
|---|---|
|
Ultrasound and Mammography |
|
|
Complete Response |
32 |
|
Not Complete Response |
120 |
|
MRI |
|
|
Complete Response |
20 |
|
Not Complete Response |
94 |
A total of 21% patients demonstrated CR as measured through ultrasound and MRI at breast and axillary lymph node, respectively.
Pathological Response
The pCR rate, defined as the total absence of invasive tumor in both breast and axillary nodes (ypT0/is ypN0), was 44.7%. The complete response rate was more in hormonal receptor–negative (93.1%) than in hormonal receptor–positive (6.9%) cancer, in grade III (86.2%) than in grade I and II (13.8%) cancer, and only 75.5% of complete response on ultrasound and MRI were also complete response on pCR. Regarding type of chemotherapy, there was no significant difference between chemotherapy with anthracycline backbone or taxane to achieve pCR (Table 2).
Surgery
At diagnosis most of the patients were indicated for mastectomy (94.7%) and only 5.3% was eligible as candidates of breast-conserving surgery. After PST, 41.4% of patients were eligible for breast-conserving surgery. Axillary dissection was performed in all of the patients even though in clinical N0. We did not do sentinel lymph node biopsy (SLNB) due to the unavailability of clip in our country and the use of methylene blue as single agent in SLND which has controversial result to detect axillary lymph node metastasis in patient after PST setting (Table3).
|
Type Surgery |
Pre-PST |
Post-PST |
|---|---|---|
|
BCS |
8 (5.3%) |
63 (41.4%) |
|
Mastectomy |
144 (94.7%) |
83 (58.6%) |
Long-term Efficacy Data
At the time of the analysis in December 2019, and with a median follow-up of 60 months, 6.67% (10/150) patients suffered from breast cancer recurrence to bone, liver, and contralateral breast; two patients deceased during follow-up due to liver and lung metastasis; and one patient deceased due to traffic accident. One patient was lost to follow-up. Despite that we were unable to demonstrate an association between pCR and better DFS with p = 0.096, HR 5.7 95.0% CI (0.73–45.52). We also observed that despite achieving similar pCR statistically, those who achieved pCR had better 5-year DFS (98.3% vs. 90.4%). Patients with hormonal receptor positive tend to have lower DFS than hormonal receptor negative; hazard ratio (HR) 6.34, 95.0% CI (1.54–26.00), and p = 0.010 (Figs. 1 and 2).
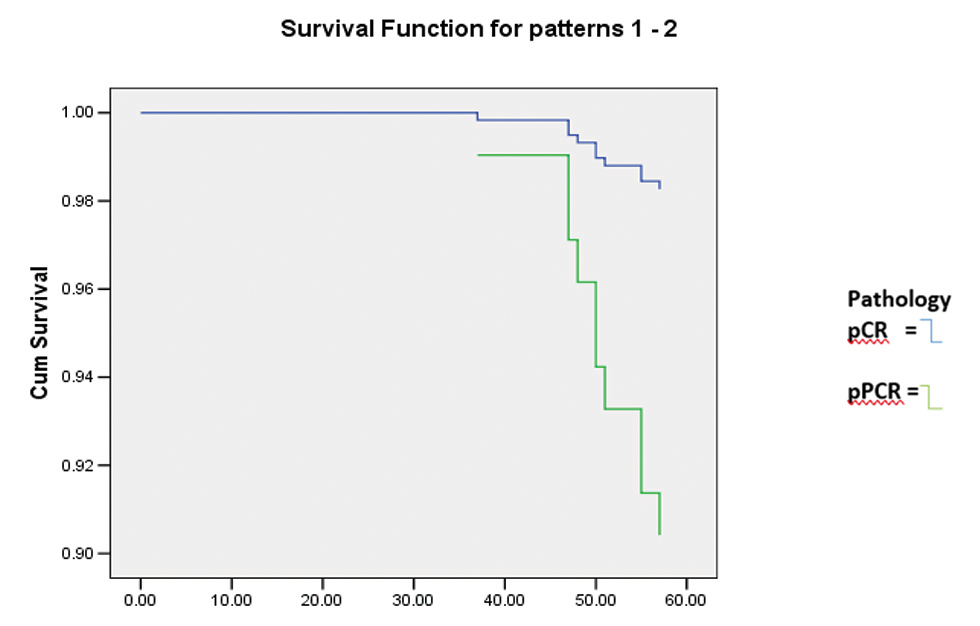
-
Fig. 1 Disease-free survival analysis bases on pathology results after primary systemic therapy assessed using Kaplan–Meier method.
-
Fig. 2 Disease-free survival analysis based on pathology result and hormonal receptor status after primary systemic therapy assessed using Kaplan–Meier method.
Cardiotoxicity
Because of the potential cardiotoxicity associated with anthracyclines and trastuzumab, detection of cardiovascular risk factors and patient monitoring were very important during the study. Age at diagnosis, body mass index (BMI), arterial hypertension, dyslipidemia, preexisting cardiovascular disease, and previous radiotherapy are recognized as risk factors for heart disease in breast cancer patients treated using anthracyclines and trastuzumab. The risk factors for developing a cardiac event in the current cohort of patients are listed in Table 4.
|
Basal risk factors for cardiac event |
Total number, n (%) |
|---|---|
|
Age at diagnosis (years) |
|
|
<50 |
117 (77.0%) |
|
50–59 |
31 (20.4%) |
|
≥ 60 |
4 (2.6%) |
|
Basal BMI |
|
|
<25 |
135 (88.8%) |
|
25–30 |
10 (6.6%) |
|
>30 |
7 (4.6%) |
|
Hypertension |
|
|
Yes |
4 (2.6%) |
|
No |
148 (97.4%) |
|
Dyslipidemia |
|
|
Yes |
19 (12.5%) |
|
No |
133 (87.5%) |
|
Type of Chemotherapy |
|
|
Anthracycline |
111(73.0%) |
|
Taxane |
41(27.0%) |
At diagnosis, all patients had normal cardiac function with an LVEF of >50%, as measured using echocardiography. The echocardiography was repeated after chemotherapy treatment and during follow-up at least 6 to 12 months after the end of adjuvant trastuzumab. At the end of chemotherapy treatment only two patients suffered a decline in LVEF < 50% (1.3% of patients). We noted some risk factors which might influence cardiac function such as dyslipidemia, hypertension, diabetes mellitus, and obesity; it did not affect meaningful LVEF decrease but it should be noted that generally the total risk factor in our population is relatively small.
Discussion
This is a retrospective cohort study in Indonesia regarding the benefit of the use of PST. Although many international guidelines declared PST for intermediate/high-risk HER2/neu-positive and triple-negative breast cancer as no longer an “option” but an ethical obligation, some issues remain controversial regarding the clear benefit of using PST for operable breast cancer in Indonesia. The balance between financial burden and benefit is the most important issue since expensive targeting therapy such as transtuzumab and pertuzumab are not under national health insurance coverage. We collected 152 patients who were fit to our criteria. After the analysis we found that pCR can be achieved at 44.7% patients and this condition added benefit for us as surgeons to do more breast-conserving surgery from 5.3% to 44.1%. This rate was quite high, compared with the previous research. We did not do SLNB and all patients underwent axillary lymph node dissection (ALND). In our institution we only used a single agent for SLND. The European and American breast society guidelines recommend using dual tracer for SLND in breast cancer after PST which converted to be N0.9,10
Even our study could not show a significant relationship between pCR with DFS; we found that hormonal receptor status clearly affected the patient’s ability to achieve pathological complete response. Similar to the other study pCR can be more expected in HER2/neu breast cancer with hormonal receptor negative. Previous study also reveals that crosstalk between the ER and HER2/neu pathways is implicated in resistance. The interactions between the estrogen receptor (ER) and HER2/neu pathways in breast cancers are clearly complex and remain incompletely understood. To the best of our knowledge, it might have contributed to the response of PST and influenced long-term remission. Cancers that express ER and HER2/neu were thought to be intrinsically resistant to endocrine therapy, likely due to HER2/neu being the dominant pathway. In fact, inhibition of HER2/neu alone in ER-positive, HER2/neu-positive breast cancer may allow ER to act as an escape pathway resulting in resistance to Her2-directed agents. Likewise, there is evidence to suggest a role of HER2/neu signaling in resistance to endocrine therapy. Given the known crosstalk between the ER and HER2/neu pathways, both in the HER2/neu-positive and HER2/neu-normal settings, a dual approach in which both pathways are inhibited concomitantly may represent an optimal therapeutic approach to overcome the potential resistance associated with targeting either pathway alone.11
Controversy exists in the assessment of the accuracy of physical examination, sonography, and mammography or even MRI in predicting the residual disease of breast tumors following the PST. Except physical examination, ultrasound (US) is used primarily for diagnostic purposes, size and to predict volume loss and determined whether BCS was suitable for the patients. As we can see in our result, US and MRI cannot be used as tools to determine whether the patient achieved complete response or not. The other problem is that it is hard for us to determine the area to excised since in Indonesia we have not had clipped yet. The only way to determine the tumor bed area was just to compare the initial imaging and imaging after PST.12
Similar to study from previous research NSABBP 27, our study also proved that using anthracycline backbone as chemotherapy agent still has clinical benefit and there is no significant difference with taxane as long as adding transtuzumab with regimen. As we mentioned earlier many of our patients cannot afford drugs due to their high cost.13For transtuzumab, there is a strict regulation regarding its use using national health insurance. Recently, we used biosimilar as the cost would be affordable for our patients. The benefit of PST can be used as surrogate marker to predict the patient who might be relapsed, becoming attractive to the authors to conduct this research. As a result, our study is consistent with the previous one in the finding that the pCR seems to have a correlation with DFS even though in this study the correlation between two groups is not significant. However, by looking at the Kaplan–Meir curve, we can still see difference between two groups, in terms of DFS.
On diagnosis, all patients had normal cardiac function with an LVEF of >50%, as measured using echocardiography. The echocardiography was repeated after chemotherapy treatment and during follow-up at least 6 to 12 months after the end of adjuvant trastuzumab. At the end of chemotherapy treatment only two patients suffered a decline in LVEF <50% (1.3% of patients). We noted some risk factor which might influence cardiac function such as dyslipidemia, hypertension, diabetes mellitus, and obesity but it did not affect meaningful LVEF decrease. However, it should be noted that generally the total risk factor in our population was relatively small compared to the same study with bigger population with complex comorbidity. For example, a study phase 3 trial was conducted by Slamon et al14 with total 469 patients affected by metastatic breast cancer who were randomly assigned to receive either a combination of doxorubicin and cyclophosphamide or paclitaxel monotherapy, both with or without trastuzumab. In this study the authors observed that ∼27% of patients in the doxorubicin and cyclophosphamide plus trastuzumab arm experienced heart failure due to cardiotoxicitu compared with 8% in the doxorubicin and cyclophosphamide alone, 13% in the paclitaxel plus trastuzumab arm, and 1% in the paclitaxel alone.
Conclusion
A sequential regimen of taxanes and anthracyclines plus trastuzumab was effective with high pCR rates and increased possibility to do BCS and had tolerable cardiotoxicity profile. It is essential to communicate to patients regarding the use of PST on HE2/neu-positive operable breast cancer nowadays not only for downstaging tumors to permit breast-conserving surgery and de-escalate surgical treatment of axilla, but also to permit clinician to monitor response therapy and provide individualized posttreatment prognostic information.
Although current guidelines have now specified recommendations for neoadjuvant treatment in HER2/neu-positive early-stage breast cancer, consensus needs to be reached in some countries such as Indonesia on breast cancer management to be in line with the international guidelines.
Acknowledgments
The authors thank all medical oncologists and surgical oncologists at Hasan Sadikin General Hospital for collecting and providing data and statistical analysis.
Conflict of Interest
None declared.
References
- Locally advanced breast cancer: treatment guideline implementation with particular attention to low- and middle-income countries. . Cancer. 2008;113(08)(01):2315-2324.
- [Google Scholar]
- Breast-conserving surgery in locally advanced breast cancer submitted to neoadjuvant chemotherapy. Safety and effectiveness based on ipsilateral breast tumor recurrence and long-term follow-up. Clinics (São Paulo). 2017;72(03):134-142.
- [Google Scholar]
- Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch Breast Cancer Audit. Eur J Surg Oncol. 2019;45(02):110-117.
- [Google Scholar]
- Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J ClinOncol. 2001;19(22):4224-4237.
- [Google Scholar]
- Efficacy and safety of neoadjuvantpertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomisedmulticentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(01):25-32.
- [Google Scholar]
- Pertuzumab plus trastuzumab in combination with standard neoadjuvantanthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(09):2278-2284.
- [Google Scholar]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(01):27-39.
- [Google Scholar]
- Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121(15):2544-2552.
- [Google Scholar]
- Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926.
- [Google Scholar]
- Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(07):609-618.
- [Google Scholar]
- Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: progress with latest therapeutic strategies. TherAdv Med Oncol. 2016;8(06):429-449.
- [Google Scholar]
- van la Parra RF, Leung JW, Yang WT. Multimodality imaging for evaluating response to neoadjuvant chemotherapy in breast cancer. AJR Am J Roentgenol. 2017;208(02):290-299.
- [Google Scholar]
- National Surgical Adjuvant Breast and Bowel Project Protocol B-27. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J ClinOncol. 2003;21(22):4165-4174.
- [Google Scholar]
- Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792.
- [Google Scholar]








