Translate this page into:
Clinical profiles and survival outcomes of adult patients with multiple myeloma at a tertiary hospital in the Philippines
Corresponding author: Dr. Jeremiah R. Vallente, Division of Hematology, Department of Medicine, University of the Philippines - Philippine General Hospital, Manila, Philippines. jeremiahrvallente@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vallente JR, Cortez CFN, Mirasol MAL. Clinical profiles and survival outcomes of adult patients with multiple myeloma at a tertiary hospital in the Philippines. Asian J Oncol, 2023;9:11.
Abstract
Objectives
The Filipino population is largely underrepresented in the currently available literature on multiple myeloma (MM). Herein, we aimed to determine the clinical profile, treatment, and outcomes of adult Filipinos with MM.
Material and Methods
The records of 74 patients with MM seen at our institution from 2016 to 2019 were retrospectively reviewed.
Results
The median age at diagnosis was 54 years, with the majority lumped in the 40–65 years age group. At diagnosis, anemia (hemoglobin <100 g/L) was present in 36 (64.3%) patients, but hypercalcemia (calcium ≥2.75 mmol/L) and azotemia (creatinine ≥177 umol/L) were seen in only 9 (20.0%) and 18 (34.0%) patients, respectively. Novel drugs (bortezomib, thalidomide, and lenalidomide) were used in 54 (84.4%) patients for frontline treatment. The overall response rate was 70.0% and the median overall survival (OS) was 60 months. On univariate analysis, only hemoglobin and the serum albumin levels affected survival.
Conclusion
Aside from the trend of a younger age at diagnosis, there are no unique clinical characteristics of MM seen in Filipinos. The longer OS may reflect the availability of newer drugs in the recent decade, but larger studies are needed to investigate the prognostic significance of several clinical and treatment parameters.
Keywords
multiple myeloma
clinical profiles
Filipino
survival outcomes
Philippines
INTRODUCTION
Multiple myeloma (MM) is a neoplastic disorder involving the clonal proliferation of plasma cells in the bone marrow. It is characterized by varying degrees and combinations of end-organ damage, which may include renal impairment, hypercalcemia, anemia, and bone destruction.[1,2] It is one of the more common hematologic malignancies, with 159,985 incident cases and 106,105 deaths annually. It accounts for 0.9% of all malignancies and 1.1% of all cancer deaths worldwide.[3] The incidence seems to vary by ethnicity, with lower incidence rates in Asians compared to people in Australasia, North America, and Western Europe. However, analysis of global data over the past few decades has shown that the largest increase in MM incidence was seen in Asian countries, particularly in China, Taiwan, and North Korea.[4] In the Philippines, MM is the 23rd most common cancer overall and the 2nd most common hematologic malignancy after the leukemias. It accounts for around 708 new cases or 0.5% of new cancer cases annually.[5]
Although the majority of the available literature on multiple myeloma is based on Western populations, the recent decades have seen the emergence of reports describing the characteristics of patients in different Asian countries.[6–8] By 2014, the first multinational study to describe the clinical profiles of Asian patients with MM was published. It pooled data from major tertiary centers in China, Hong Kong, Japan, Korea, Singapore, Taiwan, and Thailand.[9] All throughout the currently available literature, however, the Philippine population is still largely underrepresented. There is a paucity of published data regarding the clinical profiles and survival outcomes of patients with MM in the Philippines. In this study, we retrospectively reviewed data representing adult Filipino patients with multiple myeloma seen at a tertiary care hospital. We looked into the clinical profile, treatment, and outcomes of these patients.
MATERIAL AND METHODS
Study setting and population
This is a single-center, retrospective cohort study which included all adult (age ≥19 years) patients with a confirmed diagnosis of multiple myeloma, seen at the Philippine General Hospital from June 2016 to December 2019, regardless of date of diagnosis. The Philippine General Hospital is a tertiary training and referral center with a large number of patients with MM and one of the few Hematology training institutions in the country. The patient census of the institution’s Division of Hematology, from which the list of patients for inclusion was generated, was started last June 2016. Among the included patients, the earliest year of diagnosis was 2008. The medical charts of all the patients for possible inclusion were retrieved from the institution’s Medical Records Division. Patients were excluded if their medical charts were missing. The clinical and laboratory data at the time of diagnosis and the data on treatment and outcomes were collected through medical chart review. The data collection tools included only de-identified information, and the collected data were labeled with numerical identifiers, to guarantee patient anonymity and confidentiality. The study protocol was approved by the institution’s (University of the Philippines Manila) technical review and research ethics boards.
Statistical analysis
Descriptive statistics were used to summarize the patients’ clinical characteristics and the treatment regimens used. The overall response rate was computed as the proportion of patients whose treatment response were assessed to be either partial, very good, or complete. The overall survival (OS) was measured in months, from the date of diagnosis to the date of death (from any cause) or date of last encounter (outpatient consultation or inpatient admission) for living patients. Patients who were lost to follow-up were censored at the date of last encounter. Cumulative survival curves were plotted according to the Kaplan–Meier method. The log-rank test was used to compare the survival differences among categorical variables. Multivariate analysis through Cox regression was not done because of the small sample size. The precomputed minimum sample size for a multivariate Cox regression was 250 and was not met. This was computed according to the guideline provided by Peduzzi and colleagues[10] and was based on the estimated significant factors during the univariate run of five variables and the OS approximated from Kaplan–Meier curves in the study of Kim and colleagues.[6] All p-values were two-sided, with 0.05 chosen as the level of statistical significance. All statistical analyses were performed using MedCalc statistical software version 20.
RESULTS
Patient characteristics
A total of 135 patients were initially identified from the census of the Division of Hematology, but only 74 patients had retrievable medical charts and fulfilled the inclusion criteria. The clinical and laboratory characteristics of all included patients are summarized in Table 1. The median age at diagnosis of all included patients was 54 years. Most of them (86.5%) were aged 40–65 years, and 52.7% were male. A fourth (25.7%) of the patients had a cardiovascular comorbidity and less than fourth (23.0%) presented with an extramedullary plasmacytoma at baseline. Most of the patients (64.3%) had anemia (hemoglobin <100 g/L), and 18.2% had thrombocytopenia (platelet count 100 × 109/L). Hypercalcemia (serum calcium ≥2.75 mmol/L) was seen in 20.0% of patients and significant azotemia (serum creatinine ≥177 umol/L) was present in 34.0%. Almost half (46.2%) of the patients with skeletal imaging had pathologic fracture/s at baseline. Of the 39 patients with skeletal imaging results, 92.3% had bone abnormalities. Most of the patients (84.6%) with a record of baseline serum protein electrophoresis presented with a monoclonal gammopathy. Only 12 patients had serum-free light chain assay result at baseline (75% of whom had kappa light chain predominance), while only 4 patients had a record of isotype determination (all of whom had the immunoglobulin [Ig]G isotype). The serum beta-2 microglobulin level was recorded for only three patients, all of which had a level of ≥466.5 nmol/L (mean 1611.5 nmol/L). Only 12 patients had a record of baseline conventional cytogenetic studies, all reporting a normal karyotype. None of the included patients had any record of fluorescence in situ hybridization (FISH) studies.
| Distribution | Patients, n | % | |
|---|---|---|---|
| Age at diagnosis | Total N = 74 | ||
| <40 years | 6 | 8.1 | |
| 40–65 years | 64 | 86.5 | |
| >65 years | 4 | 5.4 | |
| Sex | Males | 39 | 52.7 |
| Females | 35 | 47.3 | |
| Comorbid Conditions | Cardiovascular | 19 | 25.7 |
| Pulmonary | 1 | 1.4 | |
| Metabolic | 4 | 5.4 | |
| Hematologic | 0 | 0 | |
| HIV infection | 1 | 1.4 | |
| Extramedullary plasmacytoma | Present | 17 | 23.0 |
| Absent | 57 | 77.0 | |
| Hemoglobin | Total N = 56 | ||
| ≥100 g/L | 20 | 35.7 | |
| <100 g/L | 36 | 64.3 | |
| Platelet count | Total N = 55 | ||
| >100 × 109/L | 45 | 81.8 | |
| 50–100 × 109/L | 6 | 10.9 | |
| <50 × 109/L | 4 | 7.3 | |
| White blood cell count | Total N = 56 | ||
| >10.0 × 109/L | 15 | 26.8 | |
| 5.0–10.0 × 109/L | 33 | 58.9 | |
| <5.0 × 109/L | 8 | 14.3 | |
| Serum creatinine level | Total N = 53 | ||
|
≥177 umol/L (2.0 mg/dL) |
18 | 34.0 | |
|
<177 umol/L (2.0 mg/dL) |
35 | 66.0 | |
| Serum calcium level | Total N = 45 | ||
|
≥2.75 mmol/L (11.0 mg/dL) |
9 | 20.0 | |
|
<2.75 mmol/L (11.0 mg/dL) |
36 | 80.0 | |
| Serum albumin level | Total N = 49 | ||
| ≥35 g/L | 29 | 59.1 | |
| <35 g/L | 20 | 40.8 | |
| Serum beta-2 microglobulin level | Total N = 3 | ||
|
>466.5 nmol/L (5.5 mg/L) |
3 | 100 | |
|
297–466.5 nmol/L (3.5–5.5 mg/L) |
0 | 0 | |
|
<297 nmol/L (3.5 mg/L) |
0 | 0 | |
| Plasma cells in the bone marrow | Total N = 45 | ||
| <30% | 14 | 31.1 | |
| 30–70% | 20 | 44.4 | |
| >70% | 11 | 24.4 | |
| Bone lesion/s | Total N = 39 | ||
| Pathologic fracture | 18 | 46.2 | |
| Lytic bone lesions without fracture | 16 | 41.0 | |
| Osteopenia only | 2 | 5.1 | |
| No bone lesion | 3 | 7.7 | |
| Serum protein electrophoresis | Total N = 39 | ||
| Monoclonal gammopathy | 33 | 84.6 | |
| Polyclonal gammopathy | 3 | 7.7 | |
| Normal | 3 | 7.7 | |
| Isotype | Total N = 4 | ||
| IgG | 4 | 100 | |
| IgA | 0 | 0 | |
| IgM | 0 | 0 | |
| IgD | 0 | 0 | |
| IgE | 0 | 0 | |
| Light chain | 0 | 0 | |
| Nonsecretory | 0 | 0 | |
| Serum-free light chain (FLC) assay | Total N=12 | ||
| Kappa FLC predominance | 9 | 75.0 | |
| Lambda FLC predominance | 1 | 8.3 | |
| Normal | 2 | 16.7 | |
| Cytogenetics | Total N = 12 | ||
| Normal | 12 | 100 | |
| Abnormal | 0 | 0 | |
Abbreviation: Ig, immunoglobulin.
The signs and symptoms of the patients at the time of diagnosis are summarized in Table 2. The disease most commonly presented with manifestations related to bone involvement (70.2%). The most common presenting symptom was bone pain (64.7%), mostly associated with spine involvement (46.5%). Renal manifestations (28.4%) and fatigue (25.7%) came next. Of those presenting with infection (21.6% of patients), the most commonly involved site was the respiratory system (14.9%). A notable portion of the patients (10.8%) presented with varying degrees of neurologic deficits from spinal cord compression.
| Signs and symptoms | Patients, n | % (out of 74) |
|---|---|---|
| Asymptomatic (incidental finding of anemia) | 2 | 2.7 |
| Generalized body weakness or easy fatigue | 19 | 25.7 |
| Weight loss | 4 | 5.4 |
| Manifestations of bone involvement | 52 | 70.2 |
| Bone pain | 48 | |
| Back pain | 33 | |
| Fractures | 18 | |
| Manifestations related to infection | 16 | 21.6 |
| Fever | 2 | |
| Respiratory tract infection | 11 | |
| Urinary tract infection | 1 | |
| Disseminated NTM infection | 1 | |
| Bacteremia | 1 | |
| Manifestations of kidney involvement | 21 | 28.4 |
| Proteinuria | 1 | |
| Edema | 2 | |
| Renal failure | 19 | |
| Bleeding | 4 | 5.4 |
| Epistaxis | 2 | |
| Gum bleeding | 1 | |
| Other sites of bleeding | 1 | |
| Mass or swelling | 7 | 9.5 |
| Neurologic deficits from spinal cord compression (paraplegia, paraparesis, difficulty in ambulation) | 8 | 10.8 |
Abbreviation: NTM, nontuberculous mycobacterium.
Treatment
Of the 74 included patients, only 64 had a record of receiving anti-myeloma chemotherapeutic treatment at diagnosis. A summary of their treatments and outcomes is shown in Table 3.
| Patients, n | % | ||
|---|---|---|---|
| Frontline treatment by regimen | Total N = 64 | ||
| VCD (bortezomib, cyclophosphamide, and dexamethasone) | 30 | 46.9 | |
| VD (bortezomib, dexamethasone, or another steroid) | 5 | 7.8 | |
| VMP (bortezomib, melphalan, and prednisone) | 4 | 6.3 | |
| RD (lenalidomide, dexamethasone) | 2 | 3.1 | |
| TD (thalidomide, dexamethasone, or another steroid) | 2 | 3.1 | |
| CTD (cyclophosphamide, thalidomide, and dexamethasone) | 3 | 4.7 | |
| MTP (melphalan, thalidomide, and prednisone) | 2 | 3.1 | |
| VRD (bortezomib, lenalidomide, dexamethasone) | 2 | 3.1 | |
| VTD (bortezomib, thalidomide, and dexamethasone) | 4 | 6.3 | |
| MP (melphalan, prednisone) | 9 | 14.1 | |
| CD (cyclophosphamide, dexamethasone) | 1 | 1.6 | |
| Frontline treatment by key drug | Total N = 64 | ||
| Bortezomib-based (no IMiDs) | 39 | 60.9 | |
| IMiD-based (no bortezomib) | 9 | 14.0 | |
| Bortezomib and IMiD combination | 6 | 9.4 | |
| No bortezomib or IMiD | 10 | 15.6 | |
| Response to frontline treatment | Total N = 40 | ||
| Complete response | 13 | 32.5 | |
| Very good partial response | 5 | 12.5 | |
| Partial response | 10 | 25.0 | |
| Minimal to no response | 12 | 30.0 | |
Abbreviation: IMiD, immunomodulatory drug.
The majority (60.9%) received a bortezomib-based regimen, noninclusive of any immunomodulatory drug (IMiD). The most commonly used induction treatment was the VCD (bortezomib, cyclophosphamide, and dexamethasone) regimen (46.9%) followed by the MP (melphalan and prednisone) regimen (14.1%). The use of the novel drugs (bortezomib, thalidomide, and lenalidomide) in induction treatment was observed in 84.4% of the 64 patients. Only 9.4% received a regimen combining bortezomib with an IMiD (either thalidomide or lenalidomide). Of the treated patients, only 40 had a record of treatment response assessment. Complete response was demonstrated in most of them (32.5%), while up to 30.0% had minimal to no response. The overall response rate, with partial response or better, was 70.0%. Of the 74 included patients, no one underwent hematopoietic stem cell transplantation (HSCT).
Survival outcomes and prognostic factors
In Figure 1, the survival curve for all included patients is presented. The median OS was 60 months. Among the different parameters evaluated by univariate analysis, only the hemoglobin level and the serum albumin level had an impact on the OS [Table 4]. The OS of patients with hemoglobin ≥100 g/L was significantly better than that of those with hemoglobin <100 g/L (median OS: not reached [NR] versus 28 months; p = 0.0158; [Figure 2]). Although the median OS was NR in both groups (patients with serum albumin levels ≥3.5 g/dL and patients with levels <3.5 g/dL), those with serum albumin levels ≥3.5 g/dL had better survival than those with lower albumin levels (p = 0.0402; [Figure 3]). Neither the choice of frontline regimen nor the response to induction treatment was found to be a significant prognostic variable.
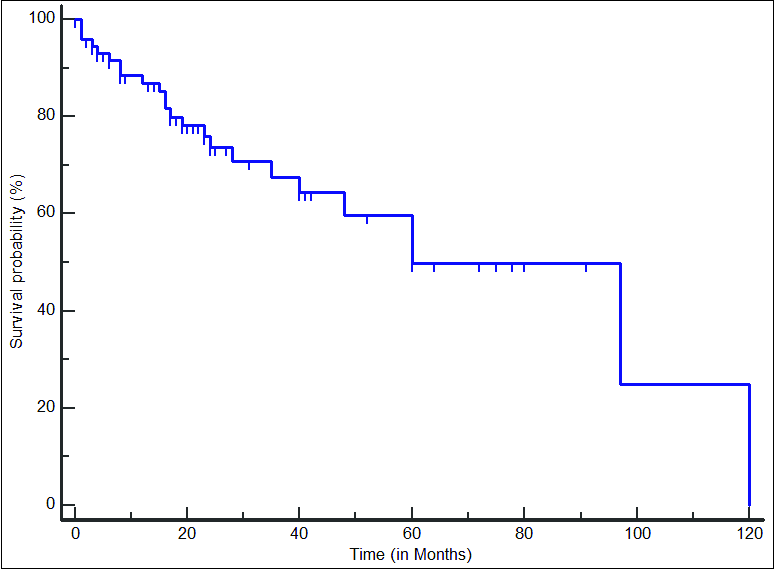
- Survival curve in 74 adult Filipino patients with multiple myeloma. The median overall survival was 60 months.
| Parameter | Patients, n (%) | Median overall survival, months | p-value | |
|---|---|---|---|---|
| Age at diagnosis | <40 years | 6 (8.1) | 48 | 0.1633 NS |
| 40–65 years | 64 (86.5) | 97 | ||
| >65 years | 4 (5.4) | 4 | ||
| Sex | Males | 39 (52.7) | 97 | 0.5450 NS |
| Females | 35 (47.3) | 60 | ||
| Extramedullary plasmacytoma | Present | 17 (23.0) | 48 | 0.3613 NS |
| Absent | 57 (77.0) | 97 | ||
| Hemoglobin | ≥100 g/L | 20 (35.7) | NR | 0.0158 |
| <100 g/L | 36 (64.3) | 28 | ||
| Platelet count | >100 × 109/L | 45 (81.8) | NR | 0.0985 NS |
| 50–100 × 109/L | 7 (10.9) | NR | ||
| <50 × 109/L | 4 (7.3) | 1 | ||
| Serum creatinine level | ≥177 umol/L (2.0 mg/dL) | 18 (34.0) | NR | 0.6196 NS |
| <177 umol/L (2.0 mg/dL) | 35 (66.0) | NR | ||
| Serum calcium level | ≥2.75 mmol/L (11.0 mg/dL) | 9 (20.0) | NR | 0.0979 NS |
| <2.75 mmol/L (11.0 mg/dL) | 36 (80.0) | NR | ||
| Serum albumin level | ≥35 g/L | 29 (59.1) | NR | 0.0402 |
| <35 g/L | 20 (40.8) | NR | ||
| Bone lesions | Pathologic fracture | 18 (46.2) | NR | 0.5633 NS |
| Lytic bone lesions without fracture | 16 (41.0) | 48 | ||
| Osteopenia or no bone lesion | 5 (12.8) | NR | ||
| Frontline treatment | Bortezomib-based | 39 (60.9) | NR | 0.0875 NS |
| IMiD-based | 9 (14.0) | 60 | ||
| Bortezomib and IMID combination | 6 (9.4) | 48 | ||
| No bortezomib or IMiD | 10 (15.6) | 97 | ||
| Response to frontline treatment | CR or VGPR | 13 (45.0) | NR | 0.6416 NS |
| PR | 10 (25.0) | NR | ||
| Minimal to no response | 12 (30.0) | NR | ||
Abbreviations: NS, not significant; NR, not reached; FLC, free light chain; IMiD, immunomodulatory drug; CR, complete response; VGPR, very good partial response; PR, partial response.
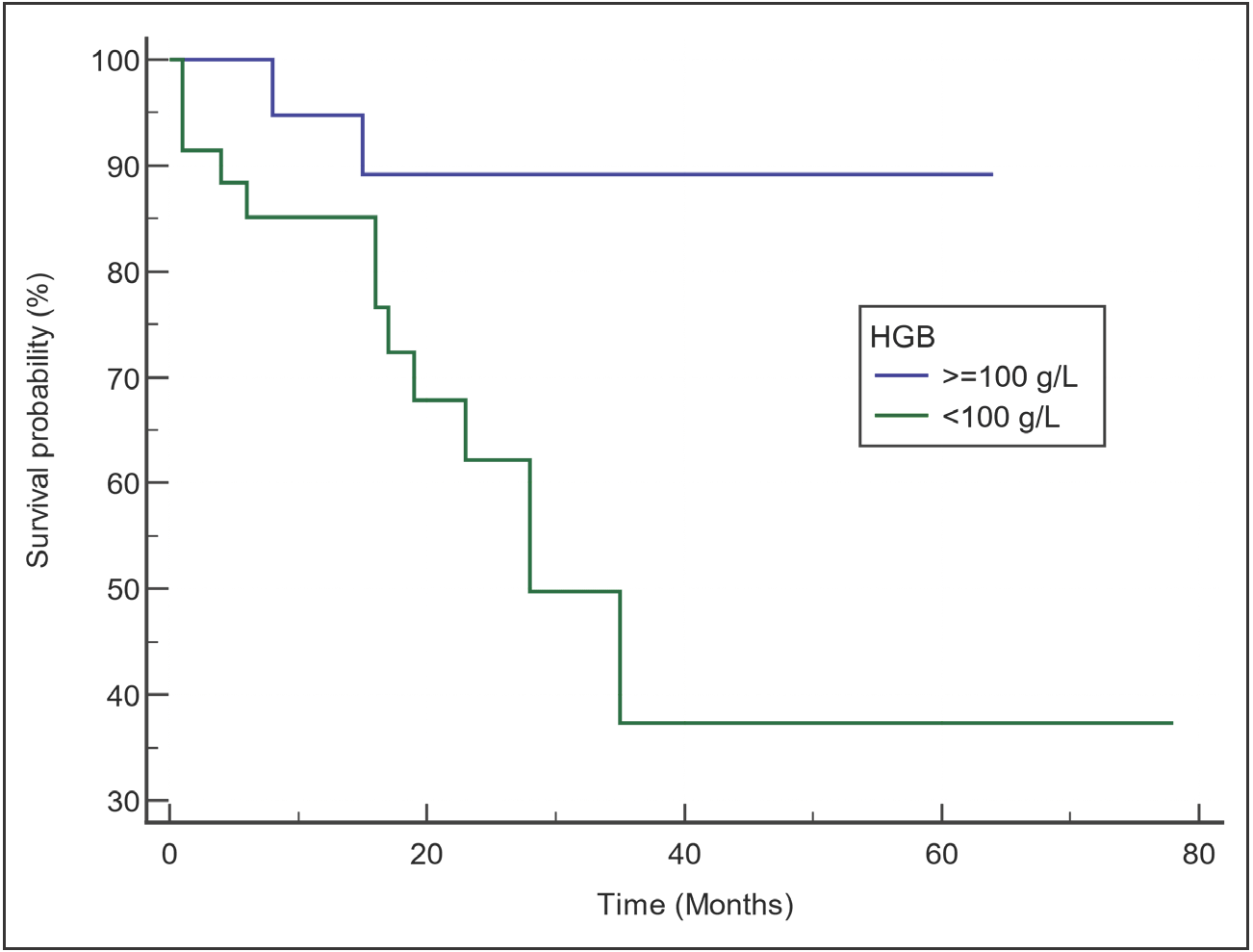
- Comparison of survival curves by hemoglobin level. Patients with hemoglobin ≥100 g/L had better survival than those with hemoglobin <100 g/L.
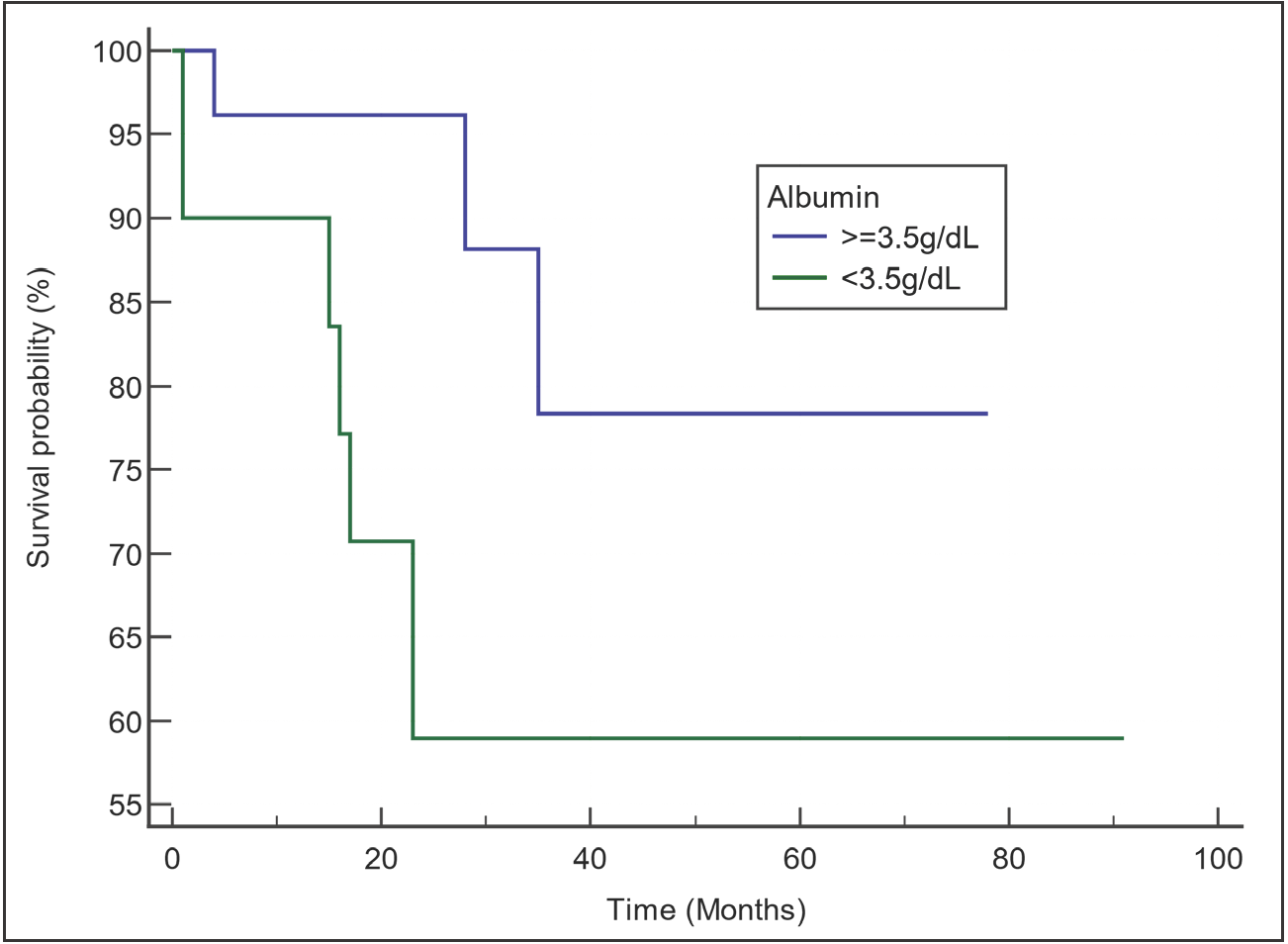
- Comparison of survival curves by serum albumin level. Median overall survival (OS) was not reached for both groups, but patients with albumin ≥35 g/L (3.5 g/dL) showed a more favorable survival than those with albumin <35 g/L (3.5 g/dL).
DISCUSSION
In this study, we looked into the demographic profile of Filipino patients with MM. Herein, slightly more males (52.7%) than females were affected with the disease. This is consistent with the data on both Western and Asian populations showing MM’s slightly higher predisposition among males.[9,11,12] The Western literature also reports MM to be a disease of the older population, with a median age of approximately 66–70 years at the time diagnosis, with only 37% of patients younger than 65 years of age.[11] Asian countries seem to have a slightly younger MM population, with the median age ranging from 59 to 66 years at the time of diagnosis,[6–9] and a higher proportion (58.5%) of patients below 65 years of age.[9] In this study, the median age was even much lower at 54 years, and the proportion of patients who were 65 years old or younger was much higher (94.6%). Consistent with the observation in the Asian Myeloma Network study, the differences in median age at diagnosis could be related to the differences in the life expectancies among different populations. The multinational study reported the highest median age at diagnosis for Japan (66), followed by Hong Kong (65), Taiwan (63), Singapore (62), Korea (61), China (59), and Thailand (59).[9] The life expectancies for these countries roughly follow the same decreasing order, with Japan having the highest life expectancy in the world at 84.2 years. The Philippines reports a much lower life expectancy (69.3 years) compared to all the aforementioned countries.[13]
We also looked into the clinical and disease characteristics of MM in the Filipino population. MM is diagnosed in a patient with clonal bone marrow plasma cell proliferation or extramedullary plasmacytoma accompanied by either an evidence of end-organ damage or the presence of a myeloma-defining biomarker. The forms of end-organ damage attributable to the disease include renal impairment, hypercalcemia, anemia, and bone lesions.[14] The myeloma-defining biomarkers include (1) a clonal bone marrow plasma cell percentage of 60% or more, (2) a ratio between the involved and uninvolved serum-free light chain of 100 or more, and (3) the presence of more than one focal bone lesion on magnetic resonance imaging studies.[14] Renal impairment can be attributable to a variety of causes (e.g. hypercalcemia, dehydration, infections, and the use of nephrotoxic agents) and is reported in up to 50% of newly diagnosed patients.[1,15,16] In this study, only 28.4% were reported to have manifestations of kidney involvement, and only 34.0% of the patients had significant azotemia. Anemia can be secondary to marrow infiltration by the proliferative plasma cells, the myelosuppression from anti-myeloma treatment, the accompanying renal damage, or a combination of these causes. It is present in nearly all patients throughout the disease course.[1,12,17] In this study, hemoglobin <100 g/L was seen in 64.3% of patients at the time of diagnosis. This value is almost twice the proportion (35%) that was observed in the Western population by Kyle and colleagues[12] but is almost similar to that (60.7%) observed by Kim and colleagues in the Asian Myeloma Network study.[9] The osteolytic bone disease from dysfunctional bone remodeling in MM can cause bone pains, pathologic fractures, and hypercalcemia.[17,18] In this study, bone abnormalities were seen in 92.3% of the patients for whom skeletal imaging was done. The proportion is higher than what was reported by Kyle and colleagues[12] (79%) and by the Asian Myeloma Network study (60.2%).[9] On the other hand, hypercalcemia was seen only in a minority of patients (20.0%), consistent with the data on both the Western and Asian populations.
Among the included patients in this study, several disease parameters were sparsely recorded. These include the serum beta-2 microglobulin levels, the myeloma isotype, and the cytogenetic profiles. Two major reasons for the paucity of these data are the cost and the availability of the laboratory tests for these parameters. Our institution is a tertiary referral and training center that caters to the financially challenged Filipinos. For these patients and their healthcare providers, the cost of diagnostics and treatment has always been an important consideration in disease management. Also, among these particular laboratory tests, only conventional cytogenetics has been readily available for years. The isotype determination by immunofixation electrophoresis was available only in the most recent years. Serum beta-2 microglobulin levels still have to be tested in other institutions. In this study, the levels were recorded in only three patients and were elevated in all of them. Isotype determination was reported in only four patients, with all of them having the IgG isotype. This does not come as a surprise since the IgG isotype is the most common, reported in around half (46.0–55.2%) of cases.[6–9,12] Although the cytogenetic profile is an important component of risk stratification in cases of MM,[19,20] it has not been consistently determined and reported in our institution. Only 12 patients in this study had a record of conventional cytogenetics result and no patient had a record of FISH testing.
The most common presenting symptom in this study was bone pain (64.7%), mostly associated with spine involvement (46.5%). This was followed by renal manifestations (28.4%) and fatigue (25.7%). Bone pain was also the most common presenting symptom among 3209 Koreans (48.4%) in a study by Kim and colleagues,[6] and among 1027 patients at the Mayo Clinic (58%) in a study by Kyle and colleagues.[12] On the contrary, Zhang and colleagues observed that fatigue was the most common presentation among 595 patients in northern China, followed only by bone pain and infection.[7]
MM has classically been described as an incurable disease. None of the established treatment modalities, including stem cell transplantation, have provided a definite cure. An enduring remission status is rather rare even after intensive interventions; the majority of patients inevitably relapse.[21,22] The survival estimates for this disease vary across different reports. In our study (year of diagnosis: 2008–2019), the median OS was 60 months, much longer than the earlier, published reports. The study on patients seen at the Mayo Clinic (year of diagnosis: 1995–1998) reported a median OS of 33 months.[12] The Asian Myeloma Network study showed a median OS of 47 months. When the patients were divided by year of diagnosis, the median OS for the 2002–2011 group was significantly longer than that of the 1986–2001 group (49 vs. 35 months).[9] Other studies from different Asian countries reported median OS ranging from 27 to 50 months.[6–8] Regardless, survival outcomes have significantly improved over the past few decades, with the rollout of several drug classes for multiple myeloma.[11,20,22,23] These include the immunomodulatory agents (IMiDs) thalidomide and lenalidomide, and the proteasome inhibitor bortezomib, all of which have been available locally in the Philippines in recent years. The last decade has also seen the approval of newer agents mostly for relapsed and refractory cases, the IMiD pomalidomide, proteasome inhibitors carfilzomib and ixazomib, and monoclonal antibodies elotuzumab, daratumumab, and isatuximab.[20] None of these newer agents, however, are locally approved and readily available in the Philippines. In this study, bortezomib-based regimens were the most commonly used treatment (60.9%) for newly diagnosed MM. Of these, the VCD regimen, used in 46.9% of patients, was the most popular. Among the included patients in this study, the earliest year of diagnosis was 2008; the same year bortezomib was first approved for frontline anti-myeloma treatment.[24] This can explain the popularity of bortezomib in this study. This practice is in contrast to that reported in earlier published studies. A study which included patients initially diagnosed with MM from 1985 to 1998 reported the use of melphalan-prednisone combination (MP regimen) in 56% of the population.[12] Neither bortezomib nor any of the IMiDs were approved for frontline myeloma treatment during that period. Thalidomide and lenalidomide received such approval last 2006 and 2014, respectively.[24] The Asian Myeloma Network study, which included patients diagnosed between 1986 and 2011, reported the use of the novel agents for frontline therapy in only 36% of 2970 patients. In this study, the novel agents were included in the induction treatment of 84.4% of the 64 patients. The rest of them were given conventional regimens (melphalan or cyclophosphamide without bortezomib or IMiD). Of these, the MP regimen was popular and second only to the VCD regimen as the most commonly used induction treatment. Despite the approval and availability of bortezomib and both IMiDs for frontline therapy, the affordability of these newer drugs was prohibitive. In the resource-limited setting of our institution, melphalan was the cheaper and more accessible alternative, especially since most of these patients were not eyeing stem cell transplantation as an eventual option.
In this study, none of the 74 patients underwent HSCT. Although autologous HSCT has been shown to increase the OS of patients and has remained a vital arm in myeloma therapy,[20,22] its availability has been widely discrepant across countries.[4] In the Philippines, centers for stem cell transplant have already been growing over the past several years but access to their services is still limited by costs and the lack of insurance coverage. In our resource-limited setting, the inaccessibility of HSCT as an eventual treatment option contributed to the popularity of frontline melphalan treatment even in supposedly transplant-eligible patients.
Across studies, factors like age, platelet count, serum albumin and creatinine levels, cytogenetic abnormalities, disease stage, and response to therapy have been shown to affect the survival of patients with multiple myeloma.[12,20] In the univariate analysis done by the Asian Myeloma Network study, even hemoglobin, marrow plasma cell percentage, and serum calcium were found to be significant prognostic factors.[9] In this current study, however, of all the parameters investigated, only the hemoglobin and the albumin levels proved be significant prognostic factors by univariate analysis. Not even the choice of frontline regimen or response to treatment affected the survival. This deviation from the widely consistent findings of much larger studies on prognostic factors in MM can be explained by the small sample size included in this study. Also, because of the sample size, only 56 patients with baseline hemoglobin levels and 49 patients with baseline albumin levels were included in the survival curves. The 18 patients with unknown hemoglobin levels and 25 patients with unknown serum albumin levels could have possibly altered the outcomes of univariate analysis if only they had a record of their laboratory results. Multivariate analysis for the prognostic factors through Cox regression was also not possible due to this limitation. A larger study in the future can verify the prognostic significance of these laboratory parameters and investigate further the impact of the other parameters on the survival outcomes of MM in the Filipino population.
CONCLUSION
In conclusion, aside from the trend of a younger age at diagnosis, there are no unique patterns in the clinical characteristics of MM in adult Filipinos when compared to that of the Western population and the rest of the Asian population. The longer median OS of 60 months may reflect the increased availability of novel anti-myeloma drugs in the recent decade. The difficult access by the majority of the Filipino population to specialized hematology tests and HSCT services is highlighted in this study. The serum albumin and hemoglobin levels remain as significant prognostic factors, but larger studies are needed to investigate the impact of other clinical and treatment parameters on the survival of adult Filipino patients with MM.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Chakraborty R, Majhail NS. Treatment and disease-related complications in multiple myeloma: implications for survivorship; 2020.
- Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol Published online 2018:1-7.
- [Google Scholar]
- International agency for research on cancer. Philippine Fact Sheets: Cancer Statistics; 2018.
- Clinical features and survival outcomes in patients with multiple myeloma: analysis of web-based data from the Korean myeloma registry. Acta Haematologica. 2009;122:200-210.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison among immunologically different subtypes of 595 untreated multiple myeloma patients in Northern China. Clin Lymphoma Myeloma Leuk. 2010;10:197-204.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of cytogenetics and interphase fluorescence in situ hybridization analysis in newly diagnosed multiple myeloma in Taiwan. Ann Oncol. 2005;16:1530-1538.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profiles of multiple myeloma in Asia — An Asian Myeloma Network study. Am J Hematol. 2014;89:751-756.
- [CrossRef] [PubMed] [Google Scholar]
- Simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma epidemiology and survival, a unique malignancy. Semin Oncol. 2017;43:676-681.
- [Google Scholar]
- Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; 2020.
- International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-e548.
- [CrossRef] [PubMed] [Google Scholar]
- Current trends of renal impairment in multiple myeloma. Kidney Dis. 2015;1:241-257.
- [Google Scholar]
- A population-based study of the impact of dialysis on mortality in multiple myeloma. Br J Haematol. 2016;10-12
- [Google Scholar]
- Guzdar A, Costello C. Supportive care in multiple myeloma. Current Hematologic Malignancy Reports. Published online 2020.
- An evidence-based approach to myeloma bone disease. Curr Hematol Malig Rep.. 2017;12:109-118.
- [CrossRef] [PubMed] [Google Scholar]
- IMWG consensus on risk stratification in multiple myeloma. Leukemia.. 2014;28:269-277.
- [CrossRef] [PubMed] [Google Scholar]
- Rajkumar SV. Multiple Myeloma: 2020 update on Diagnosis, risk-stratification and management. American Journal of Hematology. Published online 2020.
- Dramatically improved survival in multiple myeloma patients in the recent decade: results from a Swedish population-based study. Haematologica. 2018;103
- [Google Scholar]
- Recent advances in multiple myeloma: a Korean perspective. Korean J Intern Med. 2016;31:820-834.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







