Translate this page into:
Primary Squamous Cell Carcinoma of Breast Responsive to Platinum and Taxane-Based Chemotherapy: A Rare Entity and Review Literature
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pure primary squamous cell carcinoma (SCC) of the breast is a rare malignancy which constitutes < 0.1% of all primary invasive breast carcinomas. It is considered to be more aggressive compared with other infiltrating ductal cancers, and knowledge concerning treatment patterns and outcomes is limited. We treated a 50-year-old woman with SCC of left breast, having metastatic fixed axillary lymph nodes. The tumor was hormone receptor negative. Paclitaxel and carboplatin-based neoadjuvant chemotherapy was given with a good response, followed by modified radical mastectomy. Adjuvant radiotherapy was given to the chest and axilla in view of two lymph nodes positive for tumor out of 12. No local and systemic recurrence encountered in 1 year of follow-up. Surgery along with platinum and taxane-based chemotherapy and radiotherapy is an effective mode of treatment for SCC in other parts of the body. More data is necessary to formulate management guidelines, and further define if there is any role for systemic chemotherapy, radiation therapy, or hormonal blockade.
Keywords
breast cancer
squamous cell carcinoma
platinum and taxane
chemotherapy
radiotherapy
Introduction
Pure primary squamous cell carcinoma (SCC) of the breast is a rare malignancy which constitutes < 0.1% of all primary invasive breast carcinomas.1 SCC was first reported in 1908, and certain criteria need to be fulfilled before the tumor can be classified as a pure SCC of the breast. These are 1) greater than 90% of the malignant cells of squamous cell origin, 2) tumor independent of the overlying skin and nipple, 3) other sites of primary SCC excluded.2,3 Clinical and radiographic characteristics are not specific for this tumor. In general, these are very aggressive (more aggressive compared with other infiltrating ductal cancers) hormone receptor negative tumors with a poor prognosis,4 and knowledge regarding treatment patterns and outcomes is limited. The primary SCC of breast is not responsive to the traditional chemotherapeutic agents used for the breast cancers. This type of tumor is theorized to develop from squamous metaplasia in ductal carcinoma cells.5 SCC is categorized by the World Health Organization (WHO) classification of breast tumor as a purely epithelial subtype of metaplastic carcinoma and “a group of heterogeneous neoplasm characterized by a differentiation of the neoplastic epithelium into squamous cell and/or mesenchymal-looking elements” (including pure epithelial metaplastic carcinomas, squamous cell carcinoma, adenocarcinoma with spindle cell metaplasia, adenosquamous carcinoma, mucoepidermoid carcinoma, and mixed epithelial/mesenchymal metaplastic carcinomas).6
In this paper, we discussed a case of primary SCC of breast which is responsive with platinum and taxane-based chemotherapy and adjuvant radiotherapy in view of the literature.
Case Report
A 50-year-old postmenopausal woman presented at the Department of Surgical Oncology, All India Institute of Medical Sciences, Patna, with a palpable mass in her left breast and axilla for the past 1 year, gradually progressing in size, but not associated with nipple discharge and retraction or dimpling of skin. She was a multiparous lady who had breast fed all her three children and there was no family history of malignancy. She had a history of burn over the face, neck, bilateral shoulder and lower anterior trunk, sparing both breast areas, which was healed by secondary intension with skin grafting at the left shoulder region 3 years back.
Physical examination revealed a palpable 6 × 4 cm2 left breast mass located at 3 o’clock, 3 cm from the nipple. There was no associated nipple retraction, skin puckering or edema of overlying skin. There was a fixed matted lymph node of size 3 × 3 cm2 at the left axilla, with skin puckering and edema and no other palpable lymphadenopathy (cT3N2M0).
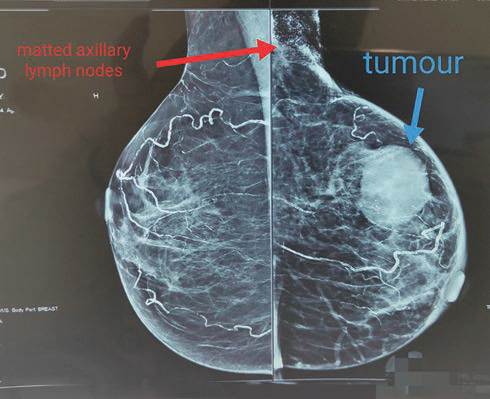
Diagnostic mammosonography revealed a well-defined round heterogenous mass lesion with central necrotic/cystic area at 3 o’clock with no abnormal clustered microcalcification, which was categorized as BIRADS 5 (Fig. 1), a large heterogenous speculated mass seen in the left axilla metastatic node.
-
Fig. 1 Left breast mammography image: well-defined round hyperdense mass in the upper outer quadrant with large heterogenous speculated mass in the left axillary region.
Ultrasound-guided core biopsy from the left breast and axillary mass was performed, which revealed moderately differentiated SCC and metastatic SCC in breast mass and axillary node, respectively.
PET/CT scan showed abnormal hypermetabolic area at the site of the left breast mass and axillary lymph nodes. Nonsuspicious nodules of lung parenchyma were found. The case was discussed at the tumor board, and paclitaxel and carboplatin-based neoadjuvant chemotherapy planned. After six cycles of chemotherapy, the size of matted axillary lymph nodes was significantly reduced, and the patient was taken up for left modified radical mastectomy (MRM).
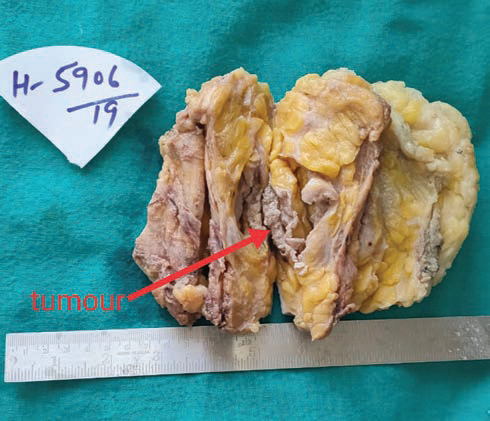
Histopathology of resected specimen showed moderately differentiated SCC of the breast, with all the margins, including deep resected margin, free of tumor. Nipple and areola complex and overlying skin was uninvolved (Figs. 23).
-
Fig. 2 Gross photograph showing intact unremarkable overlying skin and cystic tumor cavity seen after breadloafing.
-
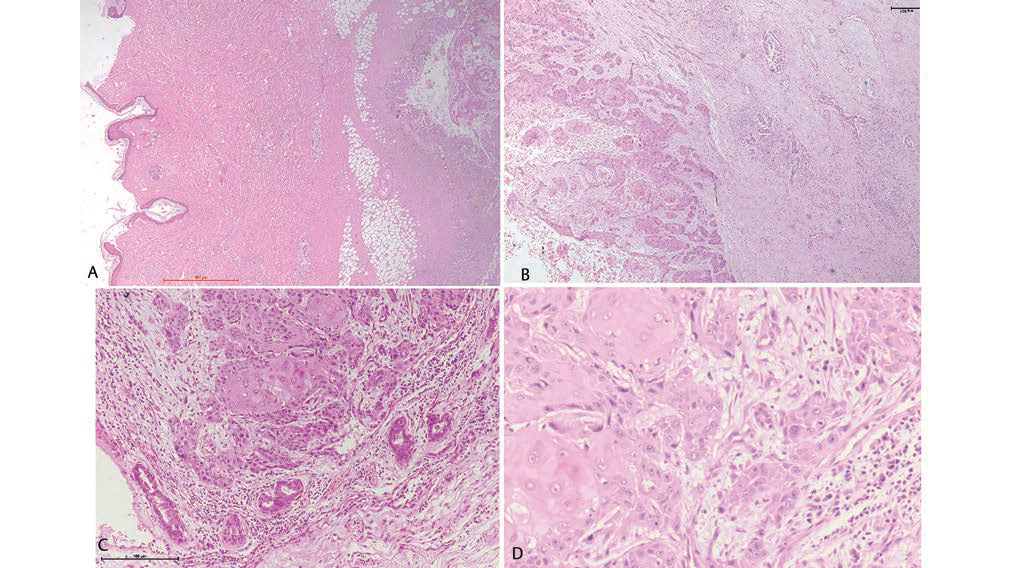
Fig. 3 (A) Unremarkable overlying skin with underlying tumor cavity (2×). (B) Tumor cavity composed of nests and cords of infiltrating squamous cell carcinoma with presence of keratin pearls (10X) along with underlying unremarkable ductal elements. (C and D) High-power view of nests of tumor cells, displaying cohesive cells, intercellular bridges, minimal pleomorphic nuclei, prominent nucleoli, mitotic figures, and moderate eosinophilic syncytial cytoplasm (20× and 40×, respectively).
Out of the 12 lymph nodes retrieved, two showed tumors (2/12) with perinodal giant cell reaction, necrosis, and cholesterol clefts. Lymphovascular invasion and perineural invasion was present. HPE: ypT3N2Mo.
Immunohistochemistry results showed a triple negative tumor (ER negative, PR negative, HER 2Nu negative). The patient received adjuvant radiotherapy at the chest wall and drainage areas (60Gy/30#/6weeks). She was followed-up with no additional treatment. One year posttreatment, the patient was locally and systemically free of disease.
Discussion
Primary SCC of breast is a very rare and aggressive type of cancer. It is not even mentioned in the classification of malignant breast tumor by standard textbooks of pathology and oncology. The histological origin of the disease is not clear. Although some authors suggest that it has originated from the breast ductal epithelium, others suggest that primary SCC of the breast may occur within squamous metaplasia of breast gland tissue.7
SCC usually presents in postmenopausal women; however, it has been reported in young women too during pregnancy and lactation.8 Our patient was postmenopausal at the time of diagnosis. Clinically, they are indistinguishable from other breast malignancies and present as a usual hard breast lump. Breast SCC are generally large (usually > 4 cm) at presentation than other breast adenocarcinomas, similar to our case which was T3 at the time of presentation.
Radiological findings are nonspecific. In mammography, microcalcifications are most frequently absent.9 Cystic lesions are characteristic presentations of SCC and more than 50% cases show cystic appearance,10 similar to our patient.
Contrary to the large primary tumor size, there is a lower rate of lymph node metastases at presentation compared with similar lesions of infiltrating duct carcinoma (IDC) (22% vs. 40–60% IDC).11 In a review of 92 cases of patients with SSC of breast, Behranwala reported that approximately 70% had no axillary nodal metastases.11 Differently, our patient had N2 disease which is not commonly seen. Hormone receptors (ER/PR) and HER2/neu are usually negative in SCC of the breast.12
Due to the low incidence and absence of adequate and accurate data, treatment of primary SCC of breast is yet to be standardized. Surgical treatment with mastectomy and axillary clearance is similar to ductal carcinoma. Breast conservative surgeries in these patients are not usually possible because most patients present with locally advanced disease.13
Rostock et al in their review suggest that SCC is not sensitive to chemotherapeutic agents commonly used for ductal carcinoma, such as cyclophosphamide, fluorouracil and adriamycin.9 Dejager et al reported that platinum-based chemotherapeutic regimen, commonly used for SCC of primary organs other than the breast, was effective for SCC of the breast also.14 Aparicio et al noted that there was no survival benefit in SCC patients who received neoadjuvant or adjuvant chemotherapy in comparison to those who did not receive chemotherapy.15 In our case, paclitaxane and carboplatin-based neoadjuvant chemotherapy was given, which was quite effective in downstaging the disease. The role of radiation is unclear. Despite the fact that SCC is generally considered to be radiosensitive,16 radiotherapy has been used only in few patients.11 Our patient received adjuvant radiotherapy in view of two nodes positive for tumor out of 12 (Table 1).
|
Study |
No. of patients |
Chemotherapeutic regime |
Types of treatment |
Result |
|---|---|---|---|---|
|
Dejager (1995)14 |
1 |
Cisplatin + 5-fluorouracil |
Neoadjuvant chemotherapy and adjuvant radiotherapy |
Complete pathological response |
|
Aparicio (2007)15 |
4 |
Cyclophosphamide + methotrexate + 5-fluorouracil (CMF) |
Adjuvant chemotherapy |
No difference in disease-free survival (DFS) and overall survival (OS) |
|
1 |
epirubicin+cyclophosphamide(ec) |
|||
|
1 |
cyclophosphamide+epirubicin+5-fluorouracil (CEF) |
|||
|
Hennessy (2005)13 |
4 |
Anthracycline/taxane-based |
Neoadjuvant chemotherapy |
No response |
|
1 |
Anthracycline-based |
|||
|
Bhatt (2009)18 |
1 |
Mitomycin C + ifosfamide + cisplatin |
Neoadjuvant radiotherapy and palliative chemotherapy (for recurrence) |
Good response |
|
Grunwald (2004)19 |
1 |
Cisplatin + paclitaxane |
Adjuvant chemotherapy and adjuvant radiotherapy |
No recurrence or relapse in 12 months follow-up |
Prognosis is controversial, although the squamous histology of breast cancer is considered very aggressive and treatment-refractory, with poor prognosis.17 Some studies demonstrated that it may have a good prognosis at an early stage. In a series of 32 patients with breast SCC, Hennessy et al reported 26% relapse-free survival rate at 5 years with localized disease. The median overall survival was 37 months (range 12–108 months) with 40% surviving at 5 years. Median survival from the time recurrent disease was recognized was 14 months (range 2–86 months).13 In the present case, however, there is no evidence of disease recurrence at 12 months.
Conclusion
The present case is rare in view of presence of axillary metastatic lymph nodes in primary SCC of breast which responded well with paclitaxane and carboplatine-based neoadjuvant chemotherapy and adjuvant radiotherapy. Primary SCC of breast is a rare and aggressive disease with no specific guidelines for the treatment. Prognosis remains unclear. Clinically, it usually presents with large primary tumor in postmenopausal women. Radiographic characteristics are not specific except the presence of cystic lesion. The tumor is hormone receptor negative. Surgery along with platinum and taxane-based chemotherapy and radiotherapy is an effective mode of treatment for SCC in other parts of the body. More data is necessary to formulate management guidelines, and further define if there is any role for systemic chemotherapy, radiation therapy, or hormonal blockade.
Conflict of Interest
None declared.
References
- Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract. 2006;202(06):465-469.
- [Google Scholar]
- Rosen’s Breast Pathology In: Chapter 21. Philadelphia, New York: Lippincott-Raven; 1997. p. :397-404.
- [Google Scholar]
- Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29(04):386-389.
- [Google Scholar]
- Primary pure squamous cell carcinoma of the breast. Clin Breast Cancer. 2005;6(03):270-272.
- [Google Scholar]
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van der Vijver MJ. World Health Organization (WHO) classification of tumours of the breast. 4th edn, vol. 4, 2012. IARC WHO Classification of Tumor, No 4.
- Squamous cell carcinoma of the breast. Eur J Obstet Gynecol Reprod Biol. 2008;137(02):222-226.
- [Google Scholar]
- Primary squamous cell carcinoma of the breast during lactation: a case report. Jpn J Clin Oncol. 2000;30(06):279-282.
- [Google Scholar]
- Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol. 2003;26(06):571-573.
- [Google Scholar]
- Breast In: Rosai J, ed. Chapter 20 In: Rosai J, ed. Ackerman’s Surgical Pathology (9th ed). St. Louis: Mosby; 2004.
- [Google Scholar]
- Primary squamous cell carcinoma of the breast: sensitivity to cisplatinum-based chemotherapy. J Surg Oncol. 1995;59(03):199-203.
- [Google Scholar]
- Squamous cell carcinoma of the breast. Eur J Obstet Gynecol Reprod Biol. 2008;137(02):222-226.
- [Google Scholar]
- Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer. 1990;65(02):272-276.
- [Google Scholar]
- Primary squamous cell carcinoma of the breast: predictors of locoregional recurrence and overall survival. Am J Surg Pathol. 2013;37(06):867-873.
- [Google Scholar]
- Primary squamous cell carcinoma of the breast: achieving long-term control with cisplatin-based chemotherapy. Clin Breast Cancer. 2009;9(03):187-188.
- [Google Scholar]
- [Primary intracystic squamous cell cancer of female breast] Zentralbl Gynäkol. 2004;126(01):36-40.
- [Google Scholar]







