Translate this page into:
Rationale, indications, techniques and applications of interstitial brachytherapy for carcinoma cervix
Address for correspondence: Dr. Indu Bansal, Department of Radiation Oncology, Max Cancer Centre, Saket, New Delhi, India. indubansal@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
With evolution of different newer radiotherapy techniques, still the role of brachytherapy in different gynecological malignancies has not sublimed. Most commonly used form of brachytherapy in carcinoma cervix patients is intracavitary brachytherapy. However, all the patients do not qualify for the treatment with intracavitary brachytherapy due to certain clinicopathological conditions. This warrants use of interstitial brachytherapy technique for treatment. For getting good results from interstitial brachytherapy, a good expertise and a proper infrastructure are needed. For perineal interstitial brachytherapy, different templates have been designed, used, and published by authors in different literature over the period. Among all these different templates, Martinez Universal Perineal Interstitial Template (MUPIT) has been used in gynecological, urological, and anorectal malignancies. In this literature review, we have discussed mainly MUPIT.
Keywords
Carcinoma cervix
interstitial brachytherapy
Martinez Universal Perineal Interstitial Template
Introduction
According to the GLOBOCAN 2012,1 cervical cancer is the fourth most common cancer in women and the seventh overall, with an estimated 528,000 new cases in 2012. There were an estimated 266,000 deaths from cervical cancer worldwide in 2012, accounting for 7.5% of all female cancer deaths. Almost 9 out of 10 (87%) cervical cancer deaths occur in the less-developed regions. Mortality varies 18-fold between the different regions of the world, with rates ranging from <2 per 100,000 in Western Asia, Western Europe, and Australia/New Zealand to >20 per 100,000 in Melanesia (20.6) and Middle (22.2) and Eastern (27.6) Africa.
In these high incidence regions, most of the cases are detected in an advanced stage, i.e., in the International Federation of Gynecology and Obstetrics (FIGO) Stage IIB-IVA.2 In the advanced stage disease, external beam radiotherapy (EBRT) along with concurrent chemotherapy followed by brachytherapy becomes the treatment of choice.2 Dose of EBRT ranges from 4000 to 5000 cGy in 20–30 fractions, 180–200 cGy per fraction over a period of 5 weeks, five fractions per week.2 This can be given using cobalt-60 machine or linear accelerator using conventional or conformal radiotherapy technique. After completion of EBRT, patients are assessed for brachytherapy. In the past, brachytherapy was given using low-dose rate sources with manual loading of sources into the patients who carried some hazards for the health-care personnel. With integration of computer-based technology with radiotherapy, afterloading techniques were developed and then remote afterloading machines were devised. Gradually, high-dose rate (HDR) sources such as iridium-192 were developed for the purpose of brachytherapy. With technological evolution of Manchester system from radium to Paris system for the present generation afterloading techniques, brachytherapy has now become much safer and less time-consuming.
In carcinoma cervix, after completion of EBRT, patients are usually subjected to intracavitary brachytherapy. In intracavitary brachytherapy, a very high dose of radiation is achieved over the cervix at the center and the reference point ‘A’ dose depends on the fractionation schedule practised, which varies from institution to institution. However, beyond point A, there will be rapid dose fall off as per the inverse square law, and the parametrium and pelvic wall will get a very minimal dose. For doing intracavitary brachytherapy application, patient’s geometry must be maintained and there should be no residual in the parametrium. Parametrial residual can be addressed by parametrial boost;3 however, if there will be a large residual at the local site with improper geometry, then the intracavitary application may become a complete failure. Hence, these are the ideal candidates for interstitial brachytherapy where multiple needles will pass through the risk area to attain a planned dose inhomogeneity according to disease burden in the area. The indications for interstitial brachytherapy in gynecological malignancies are as follows.4
Indications of Interstitial Brachytherapy
Interstitial implants with 226Ra, Cs needles, or 192Ir afterloading plastic catheters are helpful in specific clinical situations.
Carcinoma cervix
-
Carcinoma cervix IB, IIB, and above if
-
a. Distorted anatomy or poor geometry
-
b. Narrow vagina and obliterated fornices not allowing an ovoid or colpostat
-
c. Loss of endocervical canal not allowing a tandem placement
-
-
Bulky primary disease2
-
Bulky parametrial disease which will require boost
-
Extensive paravaginal (>0.5 cm) or distal vaginal involvement
-
Persistent or recurrent carcinoma cervix post-EBRT and postbrachytherapy
-
Carcinoma of the cervical stump
-
Cut through hysterectomy or prior supracervical hysterectomy
-
Presence of a fistula and/or adjacent organ invasion.5
Carcinoma endometrium
-
Local recurrence postsurgery or radiation extending beyond the confines of vaginal vault (not extending to the pelvic wall).4
Carcinoma vagina and vulva
-
Radical brachytherapy in early lesions (T1/N0)
-
Boost in addition to EBRT in large lesions (T2/3)
-
Locally confined recurrent cases.6
General Contraindications of Interstitial Brachytherapy
-
Risks for general anesthesia/epidural anesthesia due to medical reasons
-
Technically difficult to get coverage dose
-
Disease infiltrating rectovaginal septum/posterior bladder wall at the time of brachytherapy
-
Distant metastases.
Types of Interstitial Brachytherapy in Carcinoma Cervix
In this review article, we will be focussing on interstitial brachytherapy for carcinoma cervix. Interstitial brachytherapy utilizes a transperineal template through which several hollow tubes are inserted directly into tissues. A tandem and central vaginal cylinder are incorporated into the template.7 Different types of templates used are as follows:
-
Martinez Universal Perineal Interstitial Template (MUPIT)8
-
Syed-Neblett applicator9
-
Vienna applicator10
-
Hammersmith hedgehog applicator11
-
Queen Mary Hospital applicator12
-
Benidorm template (magnetic resonance imaging [MRI] compatible applicator).13
Here, we discuss the most commonly used templates, MUPIT and Syed-Neblett templates.
Martinez Universal Perineal Interstitial Template
MUPIT is an afterloading template used in the delivery of interstitial perineal brachytherapy. It is a universal applicator with facilities for using it in all types of perineal malignancies. Martinez et al.,8 in 1984, published an article regarding the use of a template for perineal interstitial brachytherapy that has been utilized to treat 78 patients with cancers of the cervix, vagina, female urethra, perineum, prostate, and anorectum with greater control in placement of sources and improved dose-volume histogram (DVH). The clinical diagram of template is shown in Figure 1 and the template in place in a patient is shown in Figure 2.
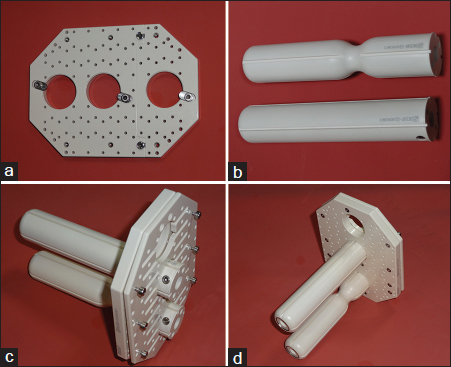
- (a) Base plate of Martinez Universal Perineal Interstitial Template. (b) Rectal and vaginal obturators. (c) Martinez Universal Perineal Interstitial Template with vaginal (middle) and rectal (lower) obturator. (d) Martinez Universal Perineal Interstitial Template with vaginal (middle) and rectal (lower) obturator
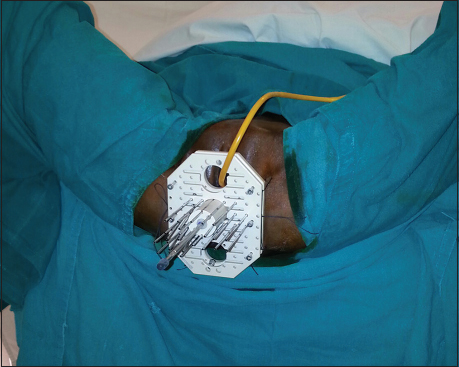
- Martinez Universal Perineal Interstitial Template placed in situ in a case of carcinoma cervix. As shown in figure, Foley catheter passes through the superior hole. Middle hole accommodated the vaginal cylinder and the inferior hole is for rectal cylinder or passage of rectal tube for intermittent suction
The applicator consists of two acrylic cylinders, one acrylic template, and a cover plate. It is a flat acrylic template with flat acrylic cover plate of size 11 cm × 8 cm × 1 cm. There are two sets of acrylic obturators, screws, and stainless steel needles. Three large holes are located along the midline. The top slot hole is for the passage of Foley’s catheter from the urethra, and the central and bottom holes are for the vaginal and rectal cylinders, respectively. There is an array of holes that for the most part determine the geometry of source placement with respect to anatomic structures. Type I holes are perpendicular to the template and only volumes extending 4 cm to either side can be covered through these holes. Type II holes are oblique or in diverging fashion to the template, angled approximately 13° laterally outward. Divergent rows allow coverage of larger volume of parametrium without hitting the ischium. Through these type II holes, volumes extending outward to 7 cm can be covered up to a depth of 14 cm. In the central hole in vaginal cylinder, there is a space for uterine tandem or hole for drainage of secretions. Each cylinder has got eight holes for placement of trocars near vaginal or rectal walls. The 17-gauge needles are stainless steel with a blinded end.14
Syed-Neblett applicator
It was originally described as “transperineal parametrial butterfly.” It is based on the same principle as MUPIT. The clinical diagram is shown in Figure 3. It has got two superimposed plastic/silicone plates each 1.2 cm thick, with 2 cm central hole for vaginal obturator, held together by screws. Predrilled holes are designed in a concentric “butterfly” pattern to accept the guide needles. There are 34 holes with rubber rings (O-rings) drilled 1 cm apart in incomplete concentric circles to accommodate the guide needles. The vaginal obturator is 2 cm in diameter and three different lengths of 12, 15, and 18 cm are available. There is a central tunnel to accommodate tandem. There are six longitudinal grooves on the surface for guide needles and embedded screw at its distal end to secure the tandem. The surface needles are used in case of anatomical distortions not allowing a proper placement of conventional intracavitary applicator.14 The 17-gauge hollow needles of 20 cm long are there for placement for interstitial brachytherapy. Applicator’s long axis is held perpendicular to sagittal plane to treat parametria.
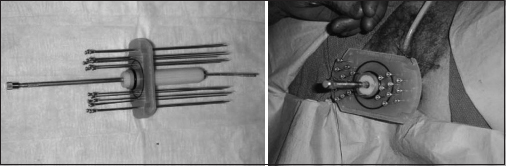
- Syed-Neblett template
Technical Modifications in Needle Placement
These templates have allowed the development of interstitial implants. Some problems however were identified from clinical experience. The needle positioning represents one of the limits in the use of such techniques. Despite the design of the templates, the parallelism of the needles is not systematically respected. The needle tips converge or diverge within the pelvic tissues. Several technical modifications have been investigated.
-
Nag et al.15 developed the use of fluoroscopy to guide the needle placement
-
Stock et al.16 have developed a technique based on transrectal ultrasound to guide the placement of needles with the Syed-Neblett template
-
Erickson et al.17 reported their experience based on computed tomography (CT)-guided needle placement in 25 patients undergoing 28 applications with advanced gynecological malignancies
-
Further investigations have evaluated the role of open MRI using specific titanium-zirconium needles by Popowski et al18
-
Apart from these technology-based noninvasive approaches, invasive surgical procedures combined with interstitial brachytherapy have been described to improve the needle placement. Disaia et al.19 reported an open laparotomy technique.
Preimplant Preparation
Use of MUPIT is an invasive procedure and is always performed in an operation theater under spinal, epidural, or general anesthesia and after maintaining thorough asepsis. Hence, a proper preoperative evaluation is to be undertaken before the procedure. A thorough history taking and documentation of any comorbidity are must before the procedure. A complete blood count, kidney function tests, liver function tests, bleeding time, clotting time, prothrombin time, international normalized ratio, X-ray of the chest posteroanterior view, and electrocardiograph/echocardiography must be done, and a proper preanesthetic evaluation has to be done in all cases. A very good bowel preparation is a must for the procedure. Enema is to be given in the evening before and in the morning of the day of intervention. The patient has to be kept fasting overnight for the procedure. The patient has to be explained about the procedure in detail and informed consent must be taken. It is always advisable that the physician performing the procedure should examine the patient and assesses the tumor extent and documents the disease extent in patient’s records with the help of clinical diagrams as per the GEC-ESTRO recommendations. It is also advisable to evaluate all the preexternal radiation treatment diseases extent clinically and radiologically and then to do a preimplant planning with the physicist a day before the procedure.
Implant Procedure
The procedure is usually performed under epidural or spinal anesthesia with full aseptic preparation. After employing thorough asepsis and administration of anesthesia, patient is kept in lithotomy position with legs strapped to the leg guards attached to the operating table. The perineal area is to be painted with povidone–iodine and clean drapes placed over abdomen, legs, perineal area and underneath the patient. Then, under good light exposure, pervaginal examination under anesthesia is performed to assess the external genitalia, vaginal length, tumor dimension, parametrial and paravaginal tissue involvement, and relationship of the tumor to the uterus and other pelvic organs as well as a thorough per-rectal examination is done. The examination should be done with double gloves and the examining pair is discarded after the examination. The inferior extent of the tumor is marked with silver markers if possible. Foley’s catheterization is done with the balloon filled with 7 ml of urografin dye.7 The cervix is then held firmly with Allis forceps and a stay suture is placed over anterior lip of the cervix or anterior fornix. The sutures are pulled through the central hole of the vaginal cylinder. The uterine sounding is done to assess the uterine length and the cervix is dilated with serial Hegar dilators. The uterine tandem is placed to verify the length of the uterine cavity with care not to perforate the fundus, with the help of ultrasound guidance. The bladder may be temporarily distended for the same by filling with 200 cc of saline for locating the fundus.20 The vaginal length is determined and the vaginal obturator is inserted over tandem until its tip abuts the cervical os. The template A is then fixed over obturator, and screws are tightened and fixed to the template at that length and skin marking is also done. The template is then fixed to the perineum by means of stitches through the peripheral holes. Ensure that the stitches knots are on the template and not on the skin as they may hurt the patient later. The distal or superior extent of the tumor determines the depth to which trocars are placed, and the proximal or inferior extension determines the active length required for adequate coverage of the implanted volume.21 A guide needle is inserted 3–4 cm beyond the clinically palpable disease, starting with the needles near the rectum, with one finger inside the rectum to avoid rectal perforation while an assistant pulls gently on the sutures. Some people place a second cylinder in the rectum and suture it to the template to ensure a fixed distance between the vagina and the rectum and to push away the posterior rectal wall. The rest of the needles are inserted around the vaginal cylinders up to the preset depth. The number and position of the needles are according to the extent of the disease and the clinical diagram prepared at the time of preimplant planning. The uro-bag should be checked repeatedly to ensure that there is no blood in the uro-bag due to bladder perforation. It is very important to do a thorough per-rectal examination after placing all the needles to confirm that no needle has pierced the rectal mucosa. The sutures initially stitched through the tumor and the normal tissues are removed. The needles are then screwed to the template, except the central needles. Now, the template B is placed over the template A. The rectal plate was attached with the rest of the template to complete the assembly. A sterile gauze soaked in betadine is placed between the template and the skin to ensure that the template does not hurt the patient. An introitus marker is also placed, and then the rectal tube is inserted through the lowermost rectal hole with a connection to an intermittent suction. The cover plate is placed over the template to prevent the needles from displacement. The T-bandage then firmly secures the template and corresponding to the position of the template; skin markings are placed on the thigh to ensure later that the template has not slipped down. The patient after getting clearance from the anesthetist is then gently transported to CT simulator ensuring throughout nondisplacement of the applicator.2
Postimplant Care
Intravenous antibiotics, hydration, parenteral nutrition, and analgesia are to be maintained properly throughout during the procedure. Daily dressing should be done and bowel sounds have to be assessed daily and should watch out for any hematuria. Patients on anticoagulation with medications as warfarin should switch to low molecular weight (LMW) heparin approximately 1 week prior to procedure, and LMW heparin may be discontinued 24 h prior to insertion time and be withheld during the duration of implant although subcutaneous heparin for thrombosis prophylaxis may be initiated after the procedure is completed.5 All precautions for prevention of deep venous thrombosis as subcutaneous heparin, compression stockings, or pneumo-boots should be undertaken. The patient should be placed on an air mattress to prevent formation of decubitus ulcers. A urinary catheter is maintained throughout treatment. The position of the different templates or applicators must be regularly checked with the help of skin markings to avoid applicator displacement.
Contouring Procedure
After placement of applicators, patient is subjected to CT scan and the scanned images are transferred to the treatment planning system. Traditionally, orthogonal radiographs were used for treatment planning, but several studies have shown potential advantages with CT or MRI simulation. CT imaging permits three-dimensional (3D) optimization of dose to the tumor with the adjacent organs at risk. MRI is better than CT scan for defining the treatment volume. It is useful to fuse CT images with MRI to take advantages of MRI while performing treatment planning on a CT simulator. It is also advisable to fuse the pretreatment MRI images with the planning scan done at the time of brachytherapy planning. The gynecological GEC-ESTRO working group issued three parts of recommendations and highlighted the pivotal role of MRI for the successful implementation of 3D image-based cervical cancer brachytherapy. The readers may refer to the GEC-ESTRO working Group IV recommendations for MRI.22 The needle positioning should be checked and adjusted to optimize their placement during CT or MRI simulation. If inadvertently some needles have gone into rectum, bladder, or bowel, it is not necessary to remove them, but care should be taken not to load them. Diluted contrast can be placed into the bladder and rectum to help with organ delineation. Critical organs such as the bladder, rectum, sigmoid, and bowel should be contoured on the axial scan. The entire organ including the wall thickness should be contoured. It is very important to accurately contour the part of the organ closest to the needles.23
The clinical target volume (CTV) is contoured as a separate structure as delineated by marker seeds, CT scan, or MRI imaging. Rather than prescribing to a point, the GEC-ESTRO working group suggested prescribing to a high-risk CTV (HR-CTV). The disease extent should be clearly documented on a clinical diagram as suggested by Hegazy et al.24 It is the gross tumour volume at the time of brachytherapy (GTVB) planning. It includes the gross disease at the time of implant as defined by T2-weighted MRI, the entire cervix, and any area clinically suspicious for gross residual disease (palpable indurations or abnormalities) or suspicious on imaging (residual “gray zones” on T2-MRI). The optimal dose to the HR-CTV remains undefined although it is common to prescribe to the same dose as would have been prescribed to point A (generally equivalent dose in 2 Gy fractions [EQD2] >80 Gy).7 For patients with a complete response or a partial responder with residual disease <4 cm, the goal should be a good coverage (i.e., a D90) with EQD2 >80 Gy. For nonresponders or those with tumors larger than 4 cm at the time of brachytherapy, tumor dose escalation to an EQD2 of 85–90 Gy is recommended to either point A or the D90 to maximize local control.25
A second volume is also defined as the intermediate risk CTV (IR-CTV). It includes the HR-CTV, the gross disease at diagnosis (GTVD), plus the additional margin of 5–15 mm, based on clinical situation. The IR-CTV should receive more than 60 Gy through dose combined by EBRT and brachytherapy.7
According to the recommendations by Hegazy et al.,24 the cranial border of the HR-CTV should be delineated at the level where uterine vessels first appear or to a point where the uterine cavity appears. Then, two slices of contour around the tandem superiorly were added to cover the cervical apex. However, it is clearly recognized in literature that for cranial tumor extension assessment, CT alone is insufficient. According to findings of a study, there may be a geographical miss in 10–35% of cases with advanced disease when defining the cranial border of HR-CTV at 2/3 or 1/2 of the uterine height. Therefore, any tailoring of this border should be avoided, if only CT is available, except in cases of very limited disease. If MRI is not available, their recommendation is, consequently, to include most of the height along the uterine cavity into the HR-CTV in advanced stages. A straightforward approach for CT-based treatment planning is to keep the full loading of intrauterine tandem length up to its tip as planning aim according to the traditional practice.24
The European study on MRI-guided brachytherapy (EMBRACE) is a multicenter trial (sponsored by the GEC-ESTRO) that will prospectively assess MRI-guided cervical brachytherapy.
Treatment Planning
Cumulative DVHs are recommended for evaluation of complex dose heterogeneity. DVH parameters for GTV, HR-CTV, and IR-CTV are the minimum dose delivered to 90% and 100% of the respective volume: D90, D100. The volume which is enclosed by 150% or 200% of the prescribed dose (V 150, V200) is recommended for overall assessment of high-dose volumes. V100 is recommended for quality assessment only within a given treatment schedule. The dose should be optimized to CTV to achieving a D90 (dose to 90% of CTV) ≥100% of the prescribed dose and at the same time minimizing dose to normal structures.26
Erickson et al.17 described a planning based on CT images allowing the definition of criteria to select an appropriate reference isodose. These criteria included: To avoid a dose rate gradient across the implant >20%, in the central plane, the isodose surface (whose value is <125% of the reference isodose) should not be contiguous and its dimensions should be <2 cm × 2 cm; the diameter of the hyper dose sleeve (two times the reference isodose) should be <1 cm. With this approach, dose rate gradients higher than 20% across the central plane of the implant were avoided in the majority of the implants. Total dose to the reference isodose ranged from 25 to 40 Gy after an external irradiation total dose of 45 Gy. The complexity of such implants also evidenced the limits of prescription points such as point A or point B and the need for further investigation including dose-volume histograms.
While assessing the late effects from brachytherapy, small volumes irradiated to higher dose seem to be of major interest. The minimum dose to bladder, rectum, sigmoid colon, vagina, and bowel in the most irradiated volume adjacent to the applicator (0.1, 1, 2, and 5 cm3) is recommended for recording and reporting. When assuming a wall thickness of 5 mm, these volumes correspond to “wall planes” of 5 mm × 4 mm (0.1 cm3), 1.4 cm × 1.4 cm (1 cm3), 2 cm × 2 cm (2 cm3), and 3.3 cm × 3.3 cm (5 cm3). It is recommended to report at least two values as 1 and 2 cm3. For practical reasons, for organ wall volumes up to 2–3 cm3, organ and organ wall contouring lead to almost identical results which allow for organ contouring only. If larger organ volumes are considered, organ wall contouring has to be performed.26
The dose distribution should be assessed in representative axial slices and adjusted by means of manual or graphical tools, to improve the coverage or normal tissue sparing. We should carefully evaluate the location and volume of hot spots, especially when using graphical optimization. In addition, it is important to review the dwell times created by optimization process. The volume of tissues receiving more than 150% of the prescription dose is limited adjacent to needles.23
In an ideal case, conformity index should be between 0.6 and 0.8. The conformation number = (CTVref/VCTV) × (CTVref/Vref) =1 (<1 practically) where CTVref is the volume of the CTV receiving a dose equal to or greater than the reference dose. It also includes the unwanted irradiated volume of critical structures outside the CTV receiving a dose equal to or greater than the reference dose.23 Similarly, the homogeneity index, defined as the fraction of the CTV receiving a dose between 100% and 150% of the reference dose, should be between 0.6 and 0.7. The volumetric evaluation of the dose distribution should be done by DVH analysis for the target volume and critical organs as well as part of optimization process.23
The total tumor dose (at 2 Gy per fraction) should be in the range of 70–85 Gy for CTV (assuming alpha-beta ratio of 10) depending on tumor location, extent of disease, and response of EBRT with 2 cm2 of rectum and sigmoid receiving ≤70–75 Gy and bladder ≤90 Gy.23 The linear quadratic biological model-EQD2 is applied for brachytherapy and is also used for calculating dose from external beam radiation.
Determining the Appropriate Dose and Fractionation Scheme
A variety of dose/fractionation schedules are used in clinical practice for HDR brachytherapy.25 For template-based implants, the entire treatment is usually done during one insertion given the complexities involved with proper placement of the brachytherapy implants. Some centers also give 2–3 insertions 1 week apart. The common-dosing schedules are given in Table 1.
|
Dose of EBRT |
Brachytherapy dose |
EQD2 (Gy) to CTV |
|---|---|---|
|
45 Gy in 25 fractions |
3.5 Gy×9 |
79.7 |
|
4.25 Gy×7 |
79.6 |
|
|
5 Gy×5 |
75.5 |
|
|
50.4 Gy in 28 fractions |
3 Gy×9 |
78.8 |
|
4.5 Gy×5 |
76.7 |
EBRT - External beam radiation therapy; EQD2 - Equivalent dose in 2 Gy fractions; CTV - Clinical target volume
Authors recommend the protocol of 5 Gy (HDR) × 5 fractions after 45 Gy/25 fractions of EBRT and 4.5 Gy (HDR) × 5 fractions after 50.4 Gy/28 fractions of EBRT. The first fraction is given on the day of implant, 2 fractions per day every 6 h apart, 2 weeks after the completion of EBRT.
Minimum Reporting Criterion for Brachytherapy
Any center following template-based brachytherapy must have a detailed reporting of the procedure.25,26 The description is given in Table 2.
|
Preprocedure checklist Initial history and clinical examination Preprocedure laboratory values as complete blood count with differential, creatinine and blood urea nitrogen, and sodium, potassium, glucose, liver function tests and PT, PTTK, INR Diagnostic imaging studies Anesthesia assessment Medication assessment (query about anticoagulants and other medications) Bowel preparation information Day before instructions given to patient as NPO or part preparation instructions Procedure checklist Consent present in the chart IV access obtained Anesthesia administered Examination under anesthesia done Complete description of clinical situation including initial history, anatomy and pathology and imaging examination dimensions and volume of GTV at diagnosis and at the time of brachytherapy, dimensions and volumes of HR CTV and IR CTV respectively Complete description of three-dimensional sectional imaging technique and contouring procedure Complete description of brachytherapy technique radionuclide Source type (wire, stepping source); source strength; applicator type; type of afterloading technique (manual or remote); description of additional interstitial needles if any; length of needle Treatment prescription and treatment planning Applicator reconstruction technique, standard loading pattern, dose specification method, optimization method if applied Prescribed dose and fractionation schedule TRAK Dose at point A (right, left, mean) D100, D90 for GTV and HR CTV and IR CTV respectively Dose to bladder and rectum for ICRU reference points D0, 1cc, D1cc, D2cc for OAR (e.g., rectum, sigmoid, bladder, vagina) D5cc, D10cc for OAR if contouring of organ wall is performed Complete description of time-dose pattern: physical and biologically weighted doses (α/ß=10 Gy for GTV and CTV; α/ß=3 Gy for OAR; T1/2=1.5 h for GTV, CTV, and OAR) QA checks Treatment delivery Applicator removed Posttreatment care and follow-up schedule |
PT - Prothrombin time; INR - International normalized ratio; GTV - Gross tumor volume; HR - High risk; CTV - Clinical target volume; IR - Intermediate risk; TRAK - Total reference air kerma; ICRU - International Commission of Radiation Unit; OAR - Organs at risk; QA - Quality assurance; PTTK - Partial thromboplastin time with kaolin; IV - Intravenous; NPO - Nil per oral
Postimplant Follow-up
All patients are kept on monthly follow-up for the first 6 months, quarterly up to 2 years, 6 monthly in the 3rd year, and annually thereafter. During follow-up, patients are evaluated for local response, complications, and distant metastasis.
Clinical Outcomes Using Image-guided Brachytherapy
There are several studies on interstitial brachytherapy using MUPIT in gynecological malignancies, a few of which are cited below as in Table 3.
|
Study |
Number of patients |
Type of implant |
EBRT dose (Gy) |
Brachy dose (Gy) |
Median follow-up (months) |
Local tumor control (%) |
Grade 3-4 late toxicity (%) |
|---|---|---|---|---|---|---|---|
|
Gupta et al.21 |
69 |
MUPIT |
39 |
32 |
30 |
60 |
14 |
|
Martinez et al.8 |
63 |
MUPIT |
- |
- |
36 |
83 |
3 |
|
Hughes-Davies et al.27 |
139 |
MUPIT |
42 |
30 |
57 |
25 |
17 |
|
Syed et al.28 |
185 |
Syed |
50.4 |
40-50 |
51 |
82 |
10 |
|
Pinn-Bingham et al.29 |
116 |
Syed |
50.4 |
36 |
35.1 |
85.3 |
13 |
EBRT - External beam radiation therapy; MUPIT - Martinez Universal Perineal Interstitial Template
As shown in Table 3, local tumor control is to a tune of 60–80% in 3 years and around 25% in 5 years with acceptable grade 3 and 4 toxicities. One of the studies done by Shrivastava et al.4 at Tata Memorial Hospital, Mumbai, showed a local control of 62% after a median follow-up of 16 months.
Nag et al.15 reported on 31 patients with carcinoma of the cervix treated with EBRT and fluoroscopically guided ISRT. With a median follow-up of 36 months, 16 patients (51%) with cervical cancer had local tumor control. The 5 years actuarial survival rate was 34%. Only 1 patient (2.5%) experienced grade 3 complication.30
Mikami et al.31 analyzed needle applicator displacement in 10 patients treated with 30 Gy HDR in 5 fractions and found on daily CT scans an average of 1–2 mm of caudal displacement. The most significant dosimetric consequences were due to changes in organ filling rather than catheter shifts.32
Sharma et al.33 presented results of 42 patients, treated from 2005 to 2007 in a prospective study of 2 weekly sessions of 10 Gy, 1 week after finishing EBRT. Median follow-up was 23 months. Delayed toxicity was 9%. The 3 years overall survival (OS) was 47% and the 3 years recurrence-free survival rates for stages IIB, IIIB, and IVA was 67%, 34%, and 20%, respectively.30
Syed et al. presented long-term results of 185 patients, treated between 1977 and 1997. All the patients were treated by a combination of external radiation 50.4 Gy to the pelvis followed by interstitial-intracavitary brachytherapy implants to a dose of 40–50 Gy to the implanted volume in two applications. Clinical local control was achieved in 82% of patients. A 5 years disease-free survival (DFS) of 65%, 67%, 49%, and 17% was achieved for patients with Stage IB, II, III, and IV disease, respectively. Eighteen (10%) patients developed RTOG grade 3 or 4 late complications.
Complications with Martinez Universal Perineal Interstitial Template Brachytherapy
Apart from surgical complications, there are a few complications of interstitial brachytherapy using MUPIT.2 They are graded as follows as shown in Table 4.
|
Grade |
Grades of Toxicities |
|---|---|
|
1 |
Mild transient symptoms of enteritis, proctosigmoiditis, cystitis, etc., that responds to conservative treatment |
|
2 |
Severe and/or persistent episodes of proctosigmoiditis, cystitis, etc., that respond to conservative treatment but requires hospitalization |
|
3 |
Fistula formation and complications that require surgical intervention |
In a study by Pinn-Bingham et al.29 on 116 patients treated between March 1996 and May 2009, after 50.4 Gy external radiation to whole pelvis, two applications of HDR-ISBT to dose of 36 Gy to implanted volume were administered. Sixty-one percent of patients also received interstitial hyperthermia, and 94 (81%) patients received chemotherapy. Clinical-locoregional control was achieved in 99 (85.3%) patients. Five years DFS rates and OS rates for entire group were 60% and 44%, respectively. The primary types of acute toxicity were grade 1 or 2 gastrointestinal and genitourinary (41.4% and 44%, respectively). Only 1 patient had grade 3 GI toxicity but no grade 4 toxicity. Approximately 13% of patients had a grade 3 late toxicity. One patient had a grade 3 colonic stricture.
Between January 2000 and December 2008, 113 patients (37 patients of cervical cancer postinadvertent surgery, 57 patients with vault cancers, and 19 patients with primary vaginal cancers) were treated at Tata Memorial Hospital, Mumbai, by Mahanshetty et al.34 with MUPIT brachytherapy boost after EBRT. The median EBRT dose was 50 Gy, median ISBT dose was 20 Gy, whereas median total dose was 73 Gy EQD2 in all three groups. Median follow-up of surviving patients for the whole group was 43 months (interquartile range, 19–67 months). The 3-year actuarial DFS and OS for three groups were 61%, 61%, 59% and 64%, 64%, and 56%, respectively. Grade 3/4 rectal toxicity was seen in 11 (10%) patients, bladder toxicity in 5 (4.5%) patients, whereas 7 (6%) patients developed grade 3 small bowel toxicity. Residual disease at brachytherapy had significant impact on DFS and OS. Other factors such as age, disease volume, parametrial extension, and vaginal extension did not impact the survivals.
Alternatives to Brachytherapy
The possible disadvantages of brachytherapy include that it is invasive, resource intensive, can be technically challenging, and is ideally performed in women who have a good performance status.7,35 Few studies are there citing comparison between brachytherapy and few of the probable options for the alternatives such as EBRT boost using intensity modulated radiotherapy (IMRT), image-guided radiotherapy, volumetric modulated arc therapy, and stereotactic body radiotherapy.36,37,38 In a study, volumes receiving 60 Gy (EQD2) were approximately twice as large for IMRT compared with brachytherapy, and the high central tumor dose was lower than that seen by brachytherapy.32 As the prior trials comparing brachytherapy and EBRT boost evidenced brachytherapy as a superior entity in terms of survival,39 these trials are mostly confined to the patients who have denied brachytherapy or not fit for undergoing brachytherapy such as bicollis or bicornis uterus and in cases of recurrence. They have also concluded that brachytherapy is the treatment of choice, but alternatives can be tried if there are constraints to brachytherapy with acceptable toxicities and comparable results. Large, prospective studies are mandatory before making any recommendation on alternatives of brachytherapy.36,37,38
Conclusion
Performing interstitial brachytherapy using implants such as MUPIT requires skill and expertise. Like other surgical procedures, high-volume centers demonstrate superior outcomes, and poor-quality implants result in less-desirable patient outcomes.40,41,42 However, the case selection for interstitial brachytherapy should be done judiciously. In cases where parametrial boost is a necessary part of the treatment or poorly maintained geometry where it is not feasible to treat with intracavitary application, interstitial brachytherapy is a good choice in terms of local control and toxicities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. et al. Available from: http://globocan.iarc.fr accessed on 06/07/2016
- MUPIT in locally advanced gynecological malignancy. J Cancer Res Ther. 2007;3:111-5.
- [Google Scholar]
- Parametrial boosting in locally advanced cervical cancer: Combined intracavitary/interstitial brachytherapy vs. intracavitary brachytherapy plus external beam radiotherapy. Brachytherapy. 2015;14:23-8.
- [Google Scholar]
- Available from http://aroi.org/ICRO_PDF/11th%20ICRO%20TMH%20 Mumbai/15.%20Brachytherapy%20perineal%20Dr.%20Shrivastava.pdf [Last updated on 2016 Jul 06; Last cited on 2016 Jul 06].
- Uterine cervix In: Halperin E C, Wazer D E, Perez C A, Brady L W, eds. Perez and Brady’s Principles and Practice of Radiation Oncology (6th ed.). Philadelphia: Lippincott William & Wilkins; 2013. p. :1484. editors.
- [Google Scholar]
- Interstitial high-dose-rate brachytherapy in locally advanced and recurrent vulvar cancer. J Contemp Brachytherapy. 2016;8:32-40.
- [Google Scholar]
- Brachytherapy in the treatment of cervical cancer: A review. Int J Womens Health. 2014;6:555-64.
- [Google Scholar]
- A multiple-site perineal applicator (MUPIT) for treatment of prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297-305.
- [Google Scholar]
- Interstitial-intracavitary (Syed-neblett) applicator in the management of carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 1979;5:95.
- [Google Scholar]
- The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624-30.
- [Google Scholar]
- A device for interstitial therapy of low pelvic tumours – The Hammersmith perineal hedgehog. Br J Radiol. 1985;58:537-42.
- [Google Scholar]
- Vaginal template implant for cervical carcinoma with vaginal stenosis or inadvertent diagnosis after hysterectomy. Int J Radiat Oncol Biol Phys. 1994;28:457-62.
- [Google Scholar]
- A new template for MRI-based intracavitary/interstitial gynecologic brachytherapy: Design and clinical implementation. J Contemp Brachytherapy. 2015;7:265-72.
- [Google Scholar]
- European Society of Therapeutic Radiation and Oncology certified [Internet]. In: “Interstitial brachytherapy in gynaecological cancer.”. The GEC ESTRO handbook of brachytherapy; 2002. Available from: http://www.estro.org/binaries/content/assets/estro/about/gec-estro/handbook-of-brachytherapy/id-17-30072002-interstitial-gyn-print_proc.pdf [Last cited on 2016 Jul 6]
- [Google Scholar]
- The use of fluoroscopy to guide needle placement in interstitial gynecological brachytherapy. Int J Radiat Oncol Biol Phys. 1998;40:415-20.
- [Google Scholar]
- A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance. Int J Radiat Oncol Biol Phys. 1997;37:819-25.
- [Google Scholar]
- CT-guided interstitial implantation of gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1996;36:699-709.
- [Google Scholar]
- Open magnetic resonance imaging using titanium-zirconium needles: Improved accuracy for interstitial brachytherapy implants? Int J Radiat Oncol Biol Phys. 2000;47:759-65.
- [Google Scholar]
- American Brachytherapy Society Cervical Cancer Recommendations Committee; American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: General principlesBrachytherapy. 2012;11:33-46.
- [Google Scholar]
- Iridium-192 transperineal interstitial brachytherapy for locally advanced or recurrent gynecological malignancies. Int J Radiat Oncol Biol Phys. 1999;43:1055-60.
- [Google Scholar]
- Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113-22.
- [Google Scholar]
- American Brachytherapy Society consensus guidelines for interstitial brachytherapy for vaginal cancer. Brachytherapy. 2012;11:68-75.
- [Google Scholar]
- High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: Impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol. 2013;52:1345-52.
- [Google Scholar]
- American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapyBrachytherapy. 2012;11:47-52.
- [Google Scholar]
- Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67-77.
- [Google Scholar]
- Parametrial interstitial brachytherapy for advanced or recurrent pelvic malignancy: The Harvard/Stanford experience. Gynecol Oncol. 1995;58:24-7.
- [Google Scholar]
- Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;54:67-78.
- [Google Scholar]
- Outcomes of high-dose-rate interstitial brachytherapy in the treatment of locally advanced cervical cancer: Long-term results. Int J Radiat Oncol Biol Phys. 2013;85:714-20.
- [Google Scholar]
- Uterine cervix In: Halperin E C, Wazer D E, Perez C A, Brady L W, eds. Perez and Brady’s Principles and Practice of Radiation Oncology (6th ed.). Philadelphia: Lippincott William and Wilkins; 2013. p. :1413. editors.
- [Google Scholar]
- Daily computed tomography measurement of needle applicator displacement during high-dose-rate interstitial brachytherapy for previously untreated uterine cervical cancer. Brachytherapy. 2011;10:318-324.
- [Google Scholar]
- Uterine cervix In: Halperin E C, Wazer D E, Perez C A, Brady L W, eds. Perez and Brady’s Principles and Practice of Radiation Oncology (6th ed.). Philadelphia: Lippincott William & Wilkins; 2013. p. :1414. editors.
- [Google Scholar]
- High-dose rate interstitial brachytherapy using two weekly sessions of 10 Gy each for patients with locally advanced cervical carcinoma. Brachytherapy. 2011;10:242-248.
- [Google Scholar]
- Template based interstitial brachytherapy in gynaecological malignancies: A single institution experience. Brachytherapy. 2014;13:337-42.
- [Google Scholar]
- Image guided radiotherapy and brachytherapy for cervical cancer. Front Oncol. 2015;5:64. et al.
- [Google Scholar]
- Non-brachytherapy alternatives in cervical cancer radiotherapy: Why not? Appl Radiat Oncol. 2015;4:11-7.
- [Google Scholar]
- Stereotactic body radiotherapy as an alternative to brachytherapy in gynecologic cancer. Biomed Res Int. 2013;2013:898953.
- [Google Scholar]
- Should helical tomotherapy replace brachytherapy for cervical cancer? Case reportBMC Cancer. 2010;10:637.
- [Google Scholar]
- Concurrent chemoradiotherapy without brachytherapy in locally advanced cervical cancer. Iran J Cancer Prev. 2013;6:195-200.
- [Google Scholar]
- The quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128. Int J Gynecol Cancer. 2012;22:123-31.
- [Google Scholar]
- Patterns of brachytherapy practice for patients with carcinoma of the cervix (1996-1999): A patterns of care study. Int J Radiat Oncol Biol Phys. 2005;63:1083-92.
- [Google Scholar]
- Technically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervix. Gynecol Oncol. 1994;53:294-300.
- [Google Scholar]







