Translate this page into:
Concurrent T790M and L858R mutations in treatment-naïve metastatic non-small-cell lung cancer: A therapeutic challenge – Current treatment strategies and promising therapies of the future in a nutshell
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
De novo (pretreatment) epidermal growth factor receptor T790M mutation in non-small-cell lung cancer (NSCLC) is rare when detected by standard genotyping methods. We present a case of concurrent de novo T790M and L858R mutations detected by direct sequencing in treatment-naïve metastatic NSCLC. This case is worthy of mention as the presence of this mutation has a bearing on the choice of treatment. This article aims to evaluate the clinical outcome for metastatic NSCLC with de novo T790M mutation and formulate an optimum treatment plan in this clinical scenario. The novel targeted therapy agents have also been reviewed.
Keywords
De novo T790M mutation
L858R mutation
treatment naïve metastatic non-small-cell lung cancer
Introduction
Epidermal growth factor receptor (EGFR) T790M is the most common cause of acquired resistance to EGFR tyrosine kinase inhibitors (TKIs).1 On the other hand, de novo (pretreatment) EGFR T790M mutation is rare when detected by standard genotyping methods.2 It coexists with an activating EGFR mutation.
Here, we present a case of concurrent de novo T790M and L858R mutations in treatment-naïve metastatic non-small-cell lung cancer (NSCLC). This case is worthy of mention as the presence of this mutation has a bearing on the choice of treatment. Challenges, in this case, were to evaluate the clinical outcome for metastatic NSCLC with pretreatment T790M mutation and formulate the optimum treatment plan in this scenario.
Case Report
A 40-year-old Indian female, never-smoker, presented with low back ache and pain in the right side of the chest for 3 months. She had no comorbidities. On examination, she was found to have performance status score of 1 as per Eastern Cooperative Oncology Group. Examination of the respiratory system was within normal limits. There was tenderness in the thoracolumbar spine. She had no neurologic deficits. Rest of the systemic examination was normal.
Chest radiograph showed a coin lesion in the right mid zone. Bronchoscopy was normal. Positron emission tomography/computerized tomography (PET/CT) scanning Figure 1a and b showed a mass in the right upper lobe with a standardized uptake value (SUV) of 10.1. There were multiple bilateral lung nodules and mediastinal and hilar lymph nodes with SUVs of 5.4 and 7.6, respectively. There were metabolically active lesions in the ribs, vertebrae, manubrium and body of sternum, acetabulum of the left hip joint, and greater trochanter of the right femur. CT-guided biopsy of the lesion in the right upper lobe was suggestive of adenocarcinoma. Immunohistochemistry was positive for Napsin A, cytokeratin 7 (CK 7), and thyroid transcription factor-1. CK 20 was negative. EGFR testing by direct sequencing Figure 2 revealed two separate EGFR mutations, namely, L858R mutation in exon 21 and a de novo T790M mutation in exon 20. Anaplastic lymphoma kinase gene rearrangement was absent.
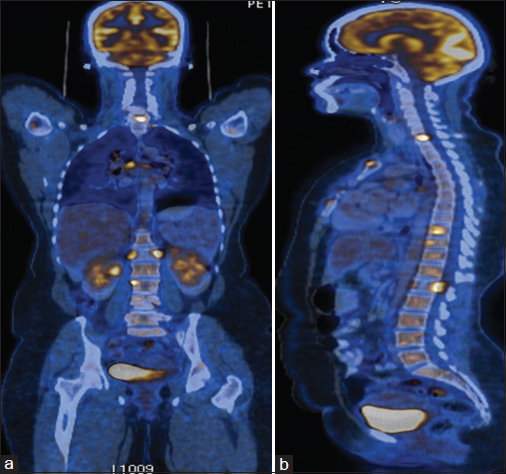
- (a and b) Positron emission tomography/computerized tomography images at baseline
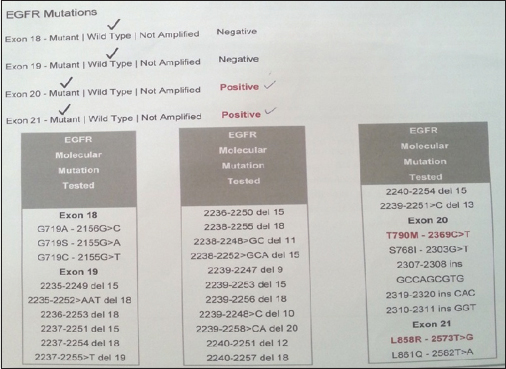
- Epidermal growth factor receptor mutation analysis by direct sequencing showing concurrent T790M and L858R mutations
She was diagnosed with adenocarcinoma lung, Stage IV, with concurrent pretreatment T790M, and L858R mutations.
We explored the different treatment options
We reviewed the literature regarding the use of erlotinib, a first generation TKI. As per the EURopean TArceva versus Chemotherapy (EURTAC) trial and a study by Su et al., those who had a de novo T790M mutation had a shorter progression-free survival (PFS) when treated with standard TKIs (erlotinib) compared with platinum-based chemotherapy Table 1.3,4
|
Study |
Type of EGFR mutation testing |
PFS |
P |
|---|---|---|---|
|
EURTAC trial erlotinib versus platinum |
Laser microdissection and peptide nucleic acid clamping PCR |
PFS With concurrent de novo T790M mutation=9.7 months Without T790M mutation=15.8 months |
0.0185 (significant) |
|
Su et al. |
Mass spectrometry |
PFS with double mutations=6.7 months With only EGFR activating mutations=10.2 months |
<0.05 |
PFS - Progression-free survival; EGFR - Epidermal growth factor receptor; EURTAC - EURopean TArceva versus Chemotherapy; PCR - Polymerase chain reaction
We then explored the role of afatinib, a second-generation irreversible TKI. Pooled analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung six trials has revealed that first-line afatinib showed response rates of 14.3% and duration of response of only 8 months for 14 patients with de novo T790M mutation, with or without activating mutations. On the other hand, those with activating mutations alone have shown a response rate of 70% and duration of response of 11 months with afatinib.5
Hence, platinum doublet chemotherapy was the treatment of choice in this scenario. She was started on pemetrexed (500 mg/m2) and carboplatin (area under the curve, 5) every 3 weeks along with zoledronic acid 4 mg every 4 weeks.
Clinical course
On day 15 of the fourth cycle of chemotherapy, she developed paraplegia. Reassessment PET/CT Figures 345 revealed progressive disease. While there was interval regression of the primary lesion in the right lung and mediastinal lymph node metastasis, there were new findings of anterior wedging of the first lumbar vertebral body with mild paraspinal soft tissue thickening. The highest SUV in the paraspinal region was 12.1 (previously 11).
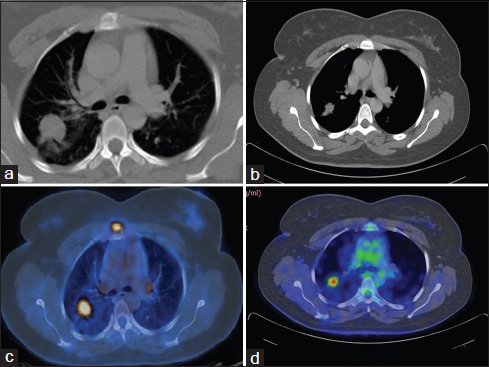
- (a and b) Baseline positron emission tomography/computerized tomography showing right upper lobe lesion (27 mm × 22 mm, standardized uptake value 11.2), mediastinal and hilar lymph nodes (standardized uptake value 7.6). (c and d) Reassessment positron emission tomography/computerized tomography after #4 showing right upper lobe lesion (16 mm × 14 mm, standardized uptake value 5.9), mediastinal and hilar lymph nodes not significant by size criteria
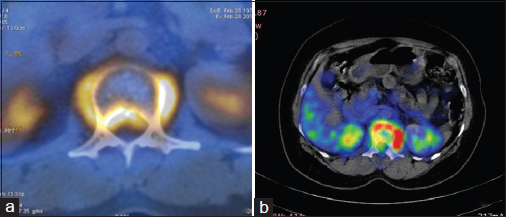
- (a) Baseline positron emission tomography/computerized tomography showing the involvement of L1 vertebra with pre- and para-vertebral soft tissue component causing spinal canal stenosis. (b) Reassessment positron emission tomography/computerized tomography after #4 showing anterior wedging of vertebral body with pre- and para-vertebral soft tissue component (standardized uptake value 12.1)
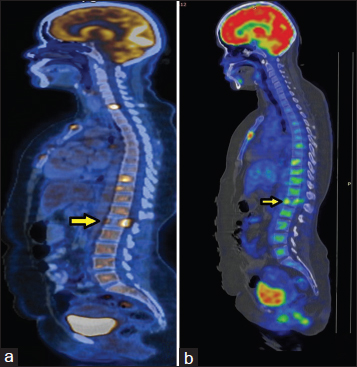
- (a) Baseline positron emission tomography: Lytic lesion in L1 vertebra, however, no wedge compression. (b) Reassessment positron emission tomography: Anterior wedging of L1 vertebra
She received palliative radiation therapy to the first lumbar vertebra (30 Gy/10 fractions). There was no improvement in the neurological deficit. She refused further treatment and opted for hospice care.
Discussion
EGFR mutations have been identified in 15% to 20% of adenocarcinomas of the lung in non-Asians and up to 50% of Asian patients.6,7,8 The incidence of EGFR mutations in the Indian population is 32%. There is a higher incidence among South Indians (47%) as compared to the North Indian population (27%).9 Treatment-naive, advanced stage adenocarcinoma patients with sensitizing EGFR mutations, have improved response rates, PFS, and quality of life.8,10,11,12,13,14,15,16,17 However, these patients inevitably develop progressive disease. This is due to acquired resistance; wherein there is the development of a second site mutation, T790M in up to 50% of the cases.18,19,20
The EGFR mutation types have been grouped according to their response to gefitinib and erlotinib. Sensitive mutations, also called responders are exon 19 deletion and exon 21 point mutation (L858R). Possible responders are exon 21 point mutation (L861Q), point mutation at exon 18, dual mutations involving exon 19 or 21, and dual mutations with exon 20 S768I. The resistant mutations, also called nonresponders are exon 20 insertion, exon 20 point mutation (T790M), and single mutation at exon 20 S768I.21
De novo T790M mutation coexists with an activating EGFR mutation. The incidence depends on the method used for detection of the mutation.22,23 When direct sequencing is used, the incidence ranges from 0.4% to 3% of all NSCLC and 1–8% of all NSCLC with an activating mutation.2 When more sensitive assays such as mass spectrometry, mutant-enriched polymerase chain reaction (PCR), laser microdissection, and peptide nucleic acid clamping PCR or colony hybridization assays are employed, it ranges from 31% to 79% of NSCLC with an activating mutation.4,24,25 It can also occur as a germline mutation which raises the possibility of genetic susceptibility and familial association.26,27,28 Patients with baseline EGFR T790M mutation have a shorter median overall survival of 16 months. This is similar to that in patients with EGFR wild-type tumors and half of what is seen in patients with sensitizing EGFR-mutant tumors.29
T790M mutation is the result of substitution of methionine for Threonine at position 790. The resultant effect of this mutation has been illustrated in Figure 6.18,30 EURTAC, LUX-Lung 2, 3, and six trials have shown that this mutation confers a poorer response to standard TKI therapy and outranks the ability of the coexisting sensitizing mutation to act as an oncogenic driver.3,4
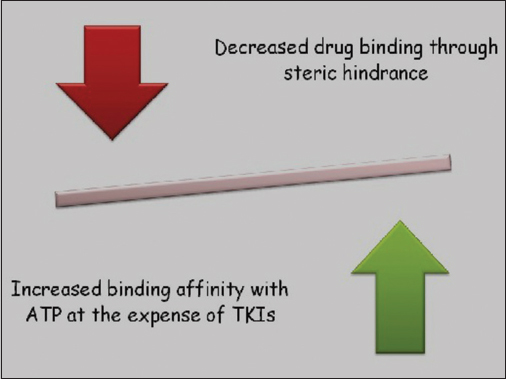
- Mechanism of action of T790M mutation
We also reviewed the literature with regard to the newer treatment options. CO-1686 (rociletinib) is a novel covalent inhibitor. It is an oral targeted therapy drug that irreversibly and selectively targets both the initial activating EGFR mutations and the T790M secondary acquired resistance mutation. In the Phase I study, and based on early findings from the ongoing Phase II trial, rociletinib yielded an overall response of 58% rate across all dose levels in trial participants with biopsy-confirmed EGFR T790M mutations. Furthermore, it did not cause the rash and diarrhea commonly associated with earlier generations of EGFR inhibitors. The important side effects (all grades) were nausea (25%), fatigue (21%), and impaired glucose tolerance/hyperglycemia (21%). Hyperglycemia was well managed with oral hypoglycemic agents and/or dose reduction.31
AZD9291 is an orally bioavailable, selective, third generation TKI, effective against both sensitive and resistant (T790M) mutations. In a Phase I study, the overall response rate was 51%. Patients with EGFR T790M positive tumors had higher overall response rates compared to those with EGFR T790M negative tumors. The most common adverse events were diarrhea, rash, and nausea.32
Dacomitinib is an oral, irreversible, small-molecule pan-HER (EGFR, HER2, and HER4) inhibitor. While preclinical studies have shown anti-tumor activity against T790M mutation-positive cell lines,33 the clinical activity of dacomitinib in patients with EGFR T790M mutation remains to be tested in larger populations. None of the four patients with EGFR exon 20 T790M mutations responded to dacomitinib in the article by Jänne PA et al.;34 however, one patient with a secondary exon 20 T790M mutation in the Japanese study by Takahashi et al. had sustained stable disease of 179 days with a 29% reduction in target lesion.35
Review of literature revealed a similar case report of concurrent de novo T790M and L858R mutation in treatment naïve advanced NSCLC by Saxena et al.36 Their patient was an 81-year-old, Chinese male, never smoker who presented with locally advanced poorly differentiated adenocarcinoma. Based on the activating mutation in exon 21, he was given erlotinib 150 mg orally daily. A PET/CT scan done 8 weeks later showed progressive disease. He had increased burden of intrapulmonary metastatic disease, worsening pleural effusion, and new lytic bone metastasis involving T5 and L1 vertebral bodies. The authors planned to discontinue erlotinib and switch over to systemic chemotherapy. However, the patient refused further treatment and opted for hospice care. This case report prompted us to opt for first-line chemotherapy for our patient. She was young, fit and had no comorbidities. Unfortunately, she experienced disease progression.
Conclusion
Drug therapy tailored for the individual patient in NSCLC should include a genetic assessment of EGFR mutational status. Erlotinib, gefitinib, and afatinib are currently available for patients with NSCLC, who harbor EGFR mutations. De novo T790M mutations confer a poor response to standard TKI therapy. As of today, cytotoxic chemotherapy may offer the best chance for response. Newer TKIs offer promise in this challenging clinical scenario.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281-9.
- [Google Scholar]
- Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. 2014;25:423-8.
- [Google Scholar]
- The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20:2001-10.
- [Google Scholar]
- Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433-40.
- [Google Scholar]
- Activity of afatinib in uncommon epidermal growth factor receptor (EGFR) mutations: Findings from three trials of afatinib in EGFR mutation-positive lung cancer. J Thorac Oncol. 2013;8:S139. et al.
- [Google Scholar]
- Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 29 et al. 18 [Abstr CRA7506].
- [Google Scholar]
- Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958-67.
- [Google Scholar]
- A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154-62.
- [Google Scholar]
- Clinical and epidemiological study of EGFR mutations and EML4-ALK fusion genes among Indian patients with adenocarcinoma of the lung. Onco Targets Ther. 2015;8:117-23.
- [Google Scholar]
- Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57.
- [Google Scholar]
- Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-8.
- [Google Scholar]
- Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-8.
- [Google Scholar]
- Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-42.
- [Google Scholar]
- Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-46.
- [Google Scholar]
- First-SIGNAL: First-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122-8.
- [Google Scholar]
- Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-34.
- [Google Scholar]
- Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-22.
- [Google Scholar]
- EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786-92.
- [Google Scholar]
- Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73.
- [Google Scholar]
- Epidermal growth factor receptor (EGFR) signaling and covalent EGFR inhibition in lung cancer. J Clin Oncol. 2012;30:3417-20.
- [Google Scholar]
- Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129-37.
- [Google Scholar]
- Concurrent molecular alterations in tumors with germ line epidermal growth factor receptor T790M mutations. Clin Lung Cancer. 2013;14:452-6.
- [Google Scholar]
- Docetaxel for non-small-cell lung cancer harboring the activated EGFR mutation with T790M at initial presentation. Onco Targets Ther. 2013;6:155-60.
- [Google Scholar]
- Highly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutation. J Thorac Oncol. 2012;7:1640-4.
- [Google Scholar]
- Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res. 2011;17:1160-8.
- [Google Scholar]
- Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315-6.
- [Google Scholar]
- Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol. 2012;7:1049-52.
- [Google Scholar]
- INHERIT EGFR – Studying Germline EGFR Mutations. ClinicalTrials.gov Identifier: NCT01754025. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01754025 [Last accessed on 2016 Dec 06].
- [Google Scholar]
- Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119:356-62.
- [Google Scholar]
- The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070-5.
- [Google Scholar]
- First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M) [abstract] J Clin Oncol. 32 et al. 05 [Abstract 8010]
- [Google Scholar]
- Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor-resistant non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 32 et al. 05 [Abstract 8009]
- [Google Scholar]
- Dacomitinib for the treatment of advanced or metastatic non-small-cell lung cancer. Future Oncol. 2014;10:813-22.
- [Google Scholar]
- Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011;17:1131-9.
- [Google Scholar]
- Phase I and pharmacokinetic study of dacomitinib (PF-00299804), an oral irreversible, small molecule inhibitor of human epidermal growth factor receptor-1, -2, and -4 tyrosine kinases, in Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30:2352-63.
- [Google Scholar]
- Double trouble: A case of concurrent de novo T790M and L858R EGFR mutations in treatment-naive advanced non-small-cell lung cancer. Oncology (Williston Park). 2014;28:526. 528, 530, 534.
- [Google Scholar]







