Translate this page into:
Carcinoma of Penis with Metastasis to Right Breast
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Penile cancer is relatively frequent in developing world compared with developed countries. Majority of histologies are of squamous origin and spread through lymphatics or vascular channels. Metastases from penile carcinoma mainly involves lung, liver, bone, and rarely other sites. The patient reported here is a treated case of penile cancer who presented with lesion in right breast after treatment for primary disease. He responded well to the adjuvant therapy.
Keywords
developing world
squamous
penile cancer
lesion in right breast
Introduction
Cutaneous metastases are external manifestations of internal malignancy. The incidence varies but can be up to 10%.1 They may appear as single or multiple, painless, small-circumscribed swellings of varied appearances or a large infiltrative mass.2 Breast, lung, gastrointestinal tract, upper aerodigestive tract, uterus, and kidney commonly contribute to skin lesions.2,3 These lesions may present after direct, lymphatic, or hematogenous spread.4 The nature of treatment offered to cutaneous metastases is mostly palliative in the form of chemotherapy, radiotherapy, local excision, laser, photodynamic therapy, cryotherapy, immune response modifier, such as imiquimod, etc.4 Whatever the treatment offers, prognosis remains poor and overall survival is typically 3 months.5
We hereby report a case of penile cancer whose breast lesion appears more than 6 months after primary surgery and postoperative radiation. To the best of our knowledge, this is the second reported case.6 The lesion in this case involved right breast on clinical and radiological examination but was proven as skin metastasis pathologically. The biopsy proved it a deposit of invasive moderately differentiated squamous cell carcinoma, not originating from overlying skin.
Case History
A 75-year-old HIV negative male, biopsy-proven case of poorly differentiated squamous cell carcinoma of glans penis extending distally to shaft with bilateral inguinal nodes, underwent total penile amputation with bilateral inguinal and iliac lymphadenectomy. On gross examination, an ulceroproliferative growth of 5 cm × 4 cm size involved penile shaft and prepuce, partially destroying the septum, and corpus cavernosum. Two out of five left and three out of nine right inguinal nodes were positive. Three left and four right iliac lymph nodes were reactive. Microscopic examination revealed a tumor infiltrating into corpus cavernosum with moderate peritumoral lymphocytic reaction and absent vascular invasion; all margins, urethra and testis free, poorly differentiated squamous cell carcinoma, and pT2N2cM0 (Stage IIIb).7 He received external beam radiotherapy to a dose of 60 Gy/33 fractions/7 weeks. The disease-free interval was 6 months.
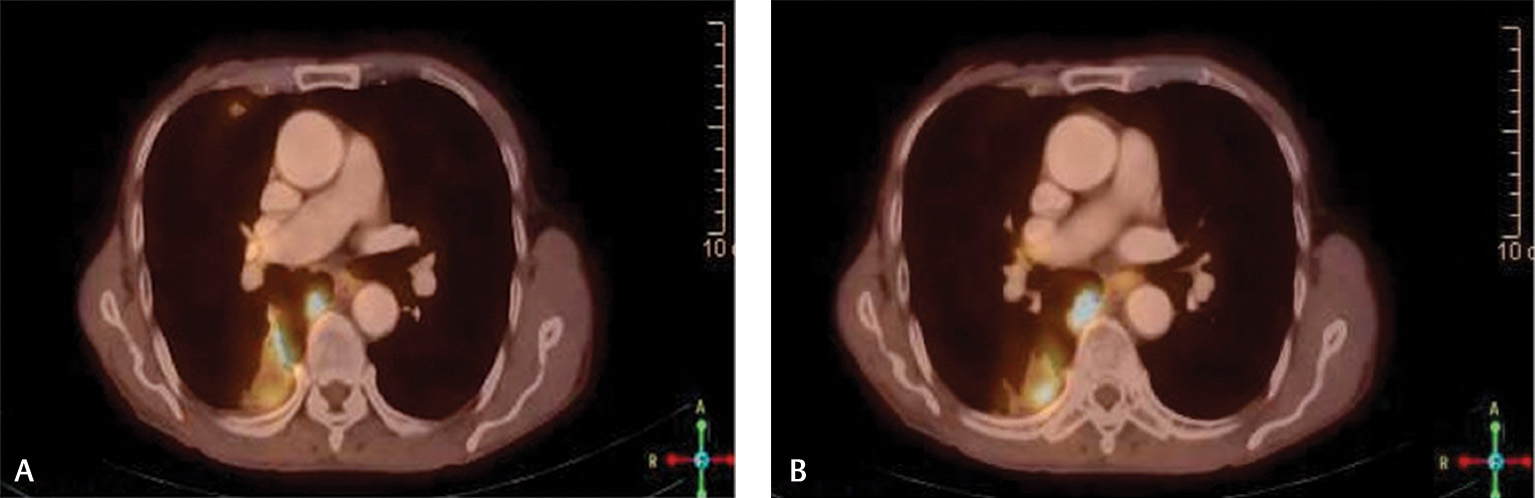
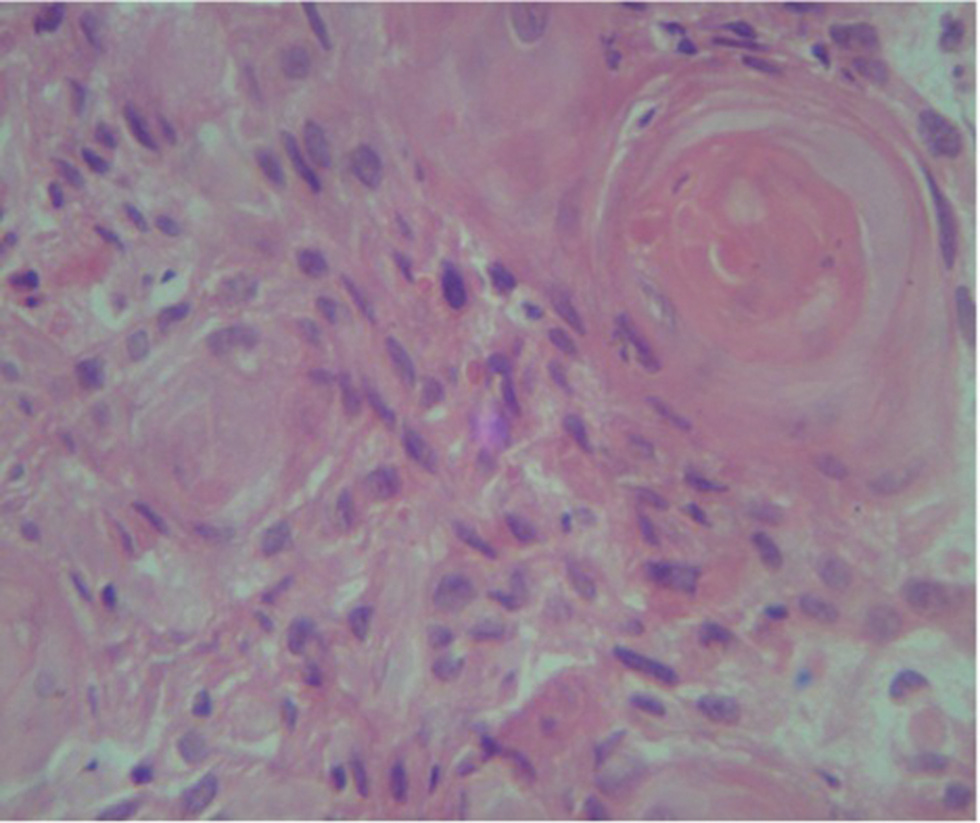
Later he presented to us with an erythematous hard periareolar swelling lying in outer quadrant of right breast since 1-month. Bilateral nipples were preserved and no axillary lymph nodes were palpable. Ultrasound showed it as abscess and fine needle aspiration cytology was negative. Within 1.5 months, it progressed to a 6 cm × 6 cm size crater shaped lesion. Whole body (WB) positron emission tomography–computed tomography (PET-CT) scan showed a metabolically active swelling in right breast, a nodular lesion in lower lobe of right lung (Fig. 1A), and a viable lesion involving bladder wall. Biopsy from breast lesion (Fig. 2) showed skin deposit of invasive moderately differentiated squamous cell carcinoma, not originating from overlying skin. Cystoscopy ruled out metastases in urinary bladder and urethra. This disease was staged as carcinoma penis post-op yprT2N2M1 (stage IV) with recurrence in skin of right breast.7 He received three weekly adjuvant chemotherapy with CEP-VB regime (cyclophosphamide 700 mg, epirubicin 60 mg, cisplatin 60 mg on day 1 and vincristine 1.5 mg, bleomycin 15 units on day 11).
-
Fig. 1 (A, B) PET-CT fusion images showing well-defined fluorodeoxyglucose avid lesion involving apical segment of lower lobe parenchyma of right lung suggestive of metastases. PET-CT, positron emission tomography–computed tomography.
-
Fig. 2 Biopsy review from breast lesion showing the characteristic “keratin pearl” deposit of invasive moderately differentiated squamous cell carcinoma, not originating from overlying skin.
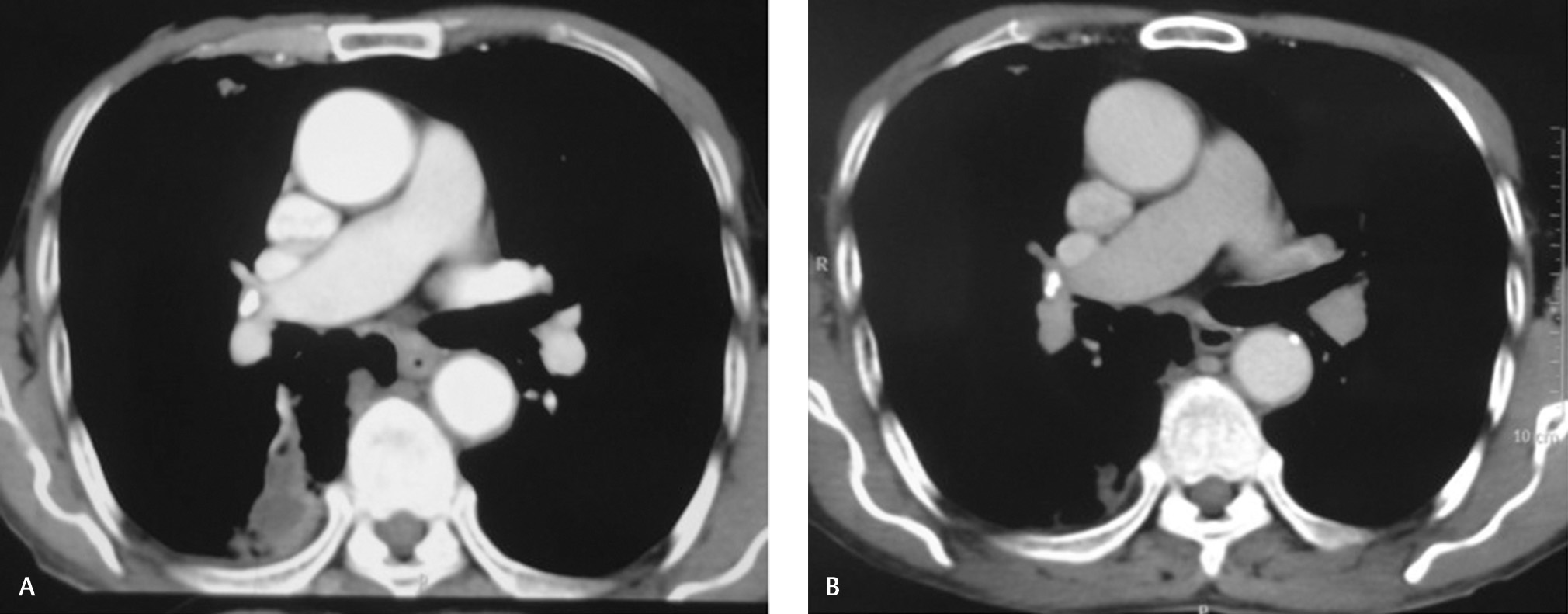
Breast mass and right lung lesions (Fig. 1) completely and metabolically resolved after four and seven cycles, respectively. He received locoregional radiation to right chest wall to a dose of 50 Gy/20 fractions/30 days along with tablet geftinib 250 mg once a day. Follow-up contrast-enhanced computed tomography (CECT) of chest, abdomen, and pelvis (Fig. 3) was normal. The patient followed-up for 1.5 years. On telephonic interrogation, he succumbed to death due to traumatic hip fracture after a total duration of 2 years.
-
Fig. 3 (A, B) Comparison of CT images at presentation and 6 months posttreatment showing resolution of metastatic lesion involving apical segment of lower lobe parenchyma of right lung. CT, computed tomography.
Discussion
The incidence of penile cancer is lowest in Jews, rare in America and Europe, but common in Asia, Africa, and South America.8 India (rural > urban) has one of the highest world incidences of penile cancer with rates up to 3.32 per 100,000 men in some regions.9 The high incidence is attributed to uncommon practice of circumcision, poor hygiene, phimosis, multiple sexual partners, human papilloma virus (HPV) and human immunodeficiency virus (HIV) infection, and exposure to tobacco.10 It is primarily an old-age disease but can occur in younger individuals as well.10 Patients present at advanced age because of embarrassment and lack of access to specialized health care.10 In our case, old age, absence of circumcision, and poor hygiene were the contributory factors.
The tumor may arise from prepuce, glans, coronal sulcus, or penile shaft.11 In our case, the tumor originated from penile shaft and involved prepuce. It can be morphologically exophytic, nodular or ulcerative.11 Our patient presented with an ulceroproliferative disease. Penile cancer can spread directly, by lymphatic or hematogenous routes.11 The rate of nodal metastases is between 40 and 50%.12 Our patient had bilateral inguinal lymph nodal metastases, both positive for malignancy and reactive bilateral iliac lymphadenopathy.
Distant metastasis in penile cancer is rare (incidence 1–10%) and occurs in presence of advanced locoregional disease.13,14 The review of literature of anecdotal reports suggests metastasis of penile cancer to lungs, liver, brain, skeleton, heart, retroperitoneum, skin of neck, and breast.6,13,15,16,17,18,19 In the first reported case of breast metastasis, the patient presented with a 2-cm irregular shaped lump, palpable near his left nipple.6 The patient reported here, presented to us with an erythematous hard periareolar swelling lying in outer quadrant of right breast. In both the cases, axillary lymph nodes were not palpable6 (present case). In our case, both the nipples were preserved.
The patient seems fairly well managed outside for primary disease with surgery and adjuvant radiation. For metastatic disease of penis, treatment has not been established because of lack of clinical trials. Combination chemotherapy seems to be an effective modality. Dexeus et al used methotrexate, bleomycin, and cisplatin combination.20 Trabulsi and Hoffman-Censits and Hakenberg and Protzel had used multiagent cisplatin-based combinations.21,22 Pizzocaro et al have used taxanes with cisplatin and fluorouracil.23 Some centers have tried targeted therapy alone or in combination with chemotherapy.21,22 Our patient was also given combination chemotherapy with CEP-VB regime to which he responded completely. He further received local chest-wall irradiation and systemic oral tyrosine kinase inhibitor.
The data review of American Cancer Society reveals 5-year relative survival rate for penile cancer with localized, regional, and distant disease as 82, 48% and not available, respectively.24 Our patient followed up with us for 1.5 years. His scans were normal. He died due to nononcological cause.
Conclusion
It can be concluded that the data pertaining to metastases from penile cancer to unusual sites is sparse and cases are limited. This case highlights the importance of rarity of disease, associated morbidity, rare site of metastasis, and use of combination chemotherapy for management. We believe that our patient fought well, in spite of having advanced disease.
The limitation of present data are that the physicians reporting this case are not the ones who encountered this case at his first visit. This is also highlighted from the fact that only the biopsy evidence of disease from the breast is available, and no clinical image can be provided. But physicians have definitely witnessed his morbidity and supported him with all the compassion and care he deserved.
Acknowledgment
The authors acknowledge Dr. Dhruv Jain, Department of Pathology, DSCI for pathological diagnosis.
Conflict of Interest
None declared.
Note
This report was presented at the 1st Indian Cancer Congress 2013, AROI (Abstract ID: 720, Genitourinary Oncology), Delhi on November 23, 2013.
References
- Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236. (2, Pt 1)
- [Google Scholar]
- Systemic disease and skin In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook’s Textbook of Dermatology. 8th ed.. Vol 3. Oxford: Wiley Blackwell Scientific Publications; 2010. p. :62.17-62.19.
- [Google Scholar]
- Penile cancer metastasizing to the breast: a case report. J Med Case Reports. 2016;10:53.
- [Google Scholar]
- Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica (41):e117.
- [Google Scholar]
- Multiple cutaneous metastases from carcinoma of the penis. J Dermatol. 2002;29(05):296-299.
- [Google Scholar]
- Penile cancer. Clinical presentation, diagnosis, and staging. Urol Clin North Am. 1992;19(02):247-256.
- [Google Scholar]
- Carcinoma of the penis metastasizing to the dorsal spine. A case report. Urol Int. 1999;62(04):249-251.
- [Google Scholar]
- Femoral metastasis from penile carcinoma: report of 2 cases. Case Rep Urol 20152015:1-3.
- [Google Scholar]
- Metastasis of penile cancer to the heart in a 20-year-old patient. Wiad Lek. 1992;45(07)Wiad Lek. 1992;45(08):314-316.
- [Google Scholar]
- Metastatic penile squamous cell carcinoma to the retroperitoneum in a man with human papillomavirus type 45. J Am Osteopath Assoc. 2008;108(06):310-312. III
- [Google Scholar]
- Combination chemotherapy with methotrexate, bleomycin and cisplatin for advanced squamous cell carcinoma of the male genital tract. J Urol. 1991;146(05):1284-1287.
- [Google Scholar]
- Chemotherapy for penile and urethral carcinoma. Urol Clin North Am. 2010;37(03):467-474.
- [Google Scholar]
- Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol. 2009;55(03):546-551.
- [Google Scholar]
- Survival Rates for Penile Cancer. Available at: https://www.cancer.org/cancer/penile-cancer/detection-diagnosis-staging/survival-rates.html Accessed November 7, 2019







