Translate this page into:
Cachexia in Cancer Patients: Systematic Literature Review
Address for correspondence Timotius I. Hariyanto, MD, Faculty of Medicine Pelita Harapan University, Boulevard Jendral Sudirman Street, Karawaci, Tangerang, Banten 15811, Indonesia. timotius.hariyanto95@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction Cachexia in cancer patients, especially in advanced stage, is recently known as an emerging problem. Cachexia occurs in about half of all patients with neoplastic disease. The diagnosis of cachexia needs comprehensive evaluation of body weight and body composition for several months. Cachexia will give negative impacts such as increased mortality, chemotoxicity, and decreased quality of life. Here, we review the current evidence describing the definition, stages, mechanisms, diagnosis and treatment of cachexia in cancer patients.
Methods We identified 75 studies and/or review articles evaluating cachexia and weight loss in cancer patients by searching PubMed and EMBASE databases.
Results Cachexia is reported across all stages and types of cancers. The most recent definition of cachexia is reported in a 2011 paper by International Consensus. The mechanism of cachexia in cancer is complex and involved many factors which elaborate together to produce cachexia. The diagnostic evaluation and cut-off measurement of cachexia, especially in cancer varied across studies. The loss of weight that happens during chemotherapy will make a poor prognosis. Cachexia can worsen chemotherapy toxicity. Combination of dietary modification and exercise with supplementation of medication that control appetite and inflammation are important in the management of cachexia in cancer patients.
Conclusion Patients with cancer are the population at risk for developing cachexia before and after chemotherapy. Cachexia diagnosis needs evaluation of body weight and body composition. Nonpharmacological treatments, such as dietary modification and physical exercise, are the best strategy to reduce cachexia in cancer patients.
Keywords
cachexia
weight loss
cancer
Introduction
Cachexia is a disorder characterized by the involuntary loss of body weight in addition to loss of homeostatic control of both energy and protein balance.1 Cachexia is associated with several chronic diseases and in particular, it can be observed as a paraneoplastic syndrome inpatients affected by cancer. Cachexia pathophysiology is associated with systemic inflammation that involved many cytokines and mediators, negative protein and energy balance, and an involuntary loss of lean body mass with lipolysis.2 Cachexia can have a profound impact on quality of life (QOL), symptom burden, and a patient’s sense of dignity. It is a very serious complication, as weight loss during cancer treatment is associated with more chemotherapy-related side effects, fewer completed cycles of chemotherapy, and decreased survivalrates.3 Cancer cachexia, at least in a mild form, occurs in approximately 50% of all patients with neoplastic disease and is a poor prognosticator.4 Importantly, more than 20% of patients with diagnosis of cancer will die due to cancer cachexia.5,6
Current therapies focus on palliation of symptoms and the reduction of distress of patients and families rather than cure.7 By combining pharmacological and nonpharmacological interventions, the multifaceted mechanisms of this complex syndrome could be addressed simultaneously, resulting in improved protein and caloric intake, gains in muscle and fat, and better physical function.
Search Strategies
A comprehensive search of literature was conducted in the PubMed (National Institute of Health [NIH]) and EMBASE database (March 1962–March 2019) using keyword combinations of the medical subject headings (MeSH) of “cachexia,” “weight loss,” “anorexia,” “body composition,” “muscle wasting,” “energy balance,” “malnutrition,” “cancer,” and “neoplasm.” Relevant reference lists were also manually searched.
Definition of Cachexia
Cachexia is defined as a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle mass with or without loss of fat mass.8 Cachexia itself has been known for centuries. Cachexia was first described by Hippocrates as “the flesh is consumed and becomes water… the abdomen fills with water, the feet and legs swell, the shoulders, clavicles, chest, and thighs melt away… The illness is fatal.”9 The term cachexia is derived from the Greek words kakós, meaning “bad things,” and hexis, meaning “condition or appearance.”10
A consensus meeting was recently held to define cachexia, finally reaching a clinical definition that can be applied in almost any clinical entity. It was eventually published in 2008. The definition that emerged is: “cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass.” The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). Anorexia, inflammation, insulin resistance and increased muscle protein breakdown are frequently associated with wasting disease. Wasting disease is distinct from starvation, age-related loss of muscle mass, primary depression, malabsorption and hyperthyroidism and is associated with increased morbidity.11
The consensus panel developed a set of diagnostic criteria to allow clinicians and researchers to make a definitive diagnosis of cachexia (Table 1).11 The key component was at least a 5% loss of edema-free bodyweight during the previous 12 months or less. The timeframe may be disease specific and is likely to be shorter in cancer (3–6 months) and longer in chronic kidney or heart failure or chronic obstructive pulmonary disease (COPD; 12 months). In cases where a history of weight loss cannot be documented, a body mass index (BMI) of <20.0 kg/m2 was considered sufficient to establish a diagnosis of cachexia.11
|
Weight loss at least 5% in 12 months or less in the presence of underlying illness, plus three of the following criteria: |
|---|
|
Abbreviation: CRP, C-reactive protein.
In 2011, an international group of experts provided the following definition of cancer cachexia: “a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that can be partially but not entirely reversed by conventional nutritional support.”12 This definition highlighted the loss of skeletal muscle mass associated with cancer cachexia and its complications including increased chemotherapy toxicity and mortality. They also offer new diagnostic criteria for cachexia in cancer patients (Table 2).12
|
Stages of Cachexia in Cancer
Cancer cachexia is a continuum (with three stages of clinical relevance: pre-cachexia, cachexia, and refractory cachexia. Not all patients traverse the entire spectrum. In precachexia, early clinical and metabolic signs (e.g., anorexia and impaired glucose tolerance) can precede substantial involuntary weight loss (i.e., ≤5%).12 The risk of progression varies and depends on factors such as cancer type and stage, the presence of systemic inflammation, low food intake, and lack of response to anticancer therapy. Patients who have more than 5% loss of stable body weight over the past 6 months, or a BMI < 20 kg/m2 and ongoing weight loss of more than 2%, or sarcopenia and ongoing weight loss of more than 2%, but have not entered the refractory stage, are classified as having cachexia.12 In refractory cachexia, the cachexia can be clinically refractory as a result of very advanced cancer (preterminal) or the presence of rapidly progressive cancer unresponsive to anticancer therapy.12 This stage is associated with active catabolism, or the presence of factors that render active management of weight-loss no longer possible or appropriate. Refractory cachexia is characterized by a low performance status (World Health Organization [WHO] score 3 or 4) and a life expectancy of less than 3 months.12
A preliminary study in cancer patients supported the proposed three–level staging system with respect to symptom burden, QOL, tolerability for chemotherapy, and mortality; however, patients in the precachectic and cachexia group behaved in a similar manner.13
Argilés et al developed a scoring system called cachexia score (CASCO) to enable proper quantitative staging of cachectic cancer patients.14 CASCO is mainly based on the following constituents: (1) body weight loss and composition, (2) inflammation/metabolic disturbances/immunosuppression, (3) physical performance, (4) anorexia, and (5) QOL. The score ranges from 0 to 100, mild cachexia (<25), moderate (>26 and <50), severe (>51 and <75), and terminal phase (>76 and up to 100). This scoring system has been validated and can be used in many cancer types with a clear advantage over previous classifications.14
Mechanism of Cachexia in Cancer
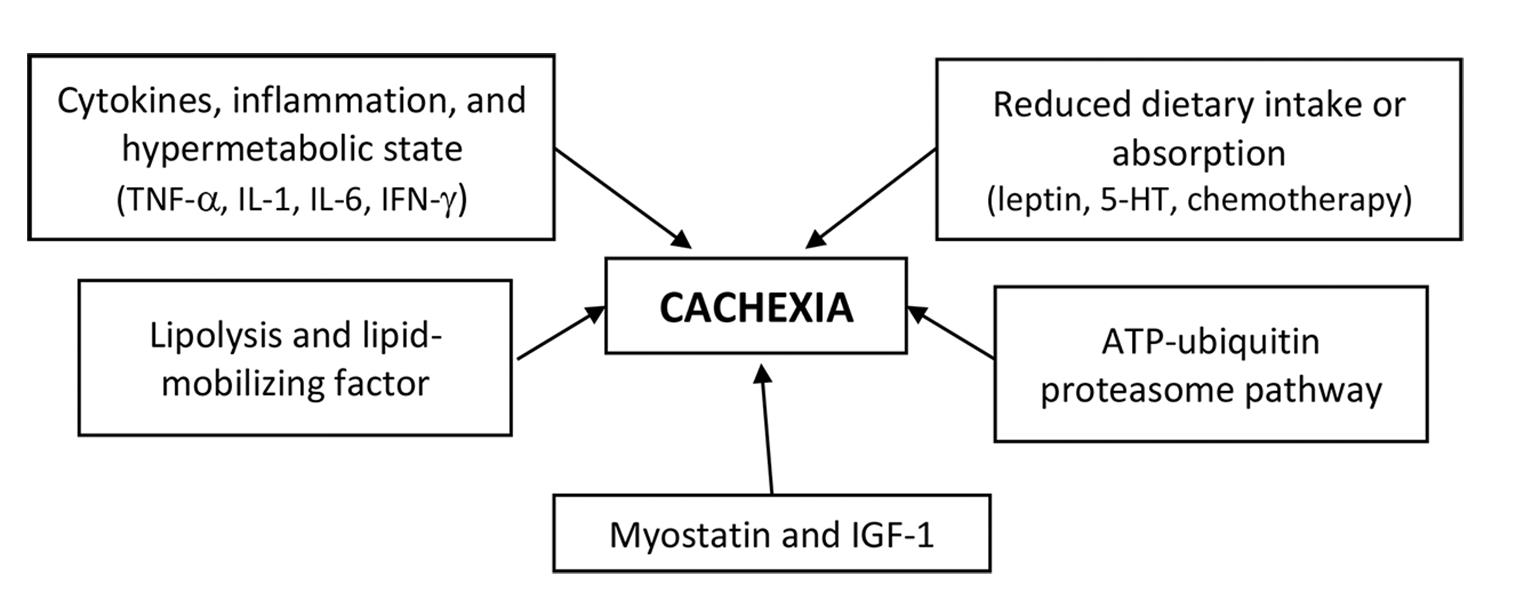
Cachexia is characterized by a combination of events. There is a negative protein and energy balance driven by a combination of reduced food intake and abnormal metabolism. There are several proposed mechanism of cancer cachexia (Fig. 1).
Cytokines, Inflammation, and Hypermetabolic State
Increases in resting energy expenditure (REE; also called basal metabolic rate) may contribute to the energy deficits that lead to wasting. An increase in REE, as measured by indirect calorimetry, has been observed in patients with lung cancer15 and sarcomas,16 and it is thought to contribute to the weight loss observed in cancer cachexia.
Numerous cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, and interferon-gamma (IFN-γ) have been postulated to play a role in the etiology of cancer cachexia.17,18,19 Cytokines activate nuclear transcription factor κB (NF-κB) that results in decreased muscle protein synthesis.20 Cytokine activation is also responsible for the reduction of MyoD protein, a transcription factor that modulates signaling pathways involved in muscle development, resulting in muscle wasting.21
Lipolysis and Lipid-Mobilizing Factor
Although wasting of lean body mass is a major aspect of cancer cachexia, loss of fat mass also occurs. A tumor-produced lipid-mobilizing factor (LMF) may contribute to wasting of fat tissue.22 It is postulated that LMF acts to sensitize adipose tissue to lipolytic stimuli by increasing cyclic adenosine monophosphate (AMP) production in adipocytes.23 This effect may be mediated through the β-adrenergic receptor, with increased receptor number or G-protein expression.22,23
The ATP–Ubiquitin–Proteasome Pathway
Activation of the adenosine triphosphate (ATP)-ubiquitin-proteasome pathway may play an important role in cancer-associated tissue wasting as illustrated by two experimental study on animal found that free ubiquitin and ubiquitin conjugates were higher in gastrocnemius muscle of tumor-implanted rats than in muscles from control rats24 and that inhibition of the ubiquitin-proteasome pathway with the proteasome inhibitor MG132 can ameliorate cachexia in tumor-bearing mice.25 Thus, the ubiquitin-proteasome pathway may be the final common pathway mediating protein degradation in cachexia.
Reduced Dietary Intake or Absorption
Anorexia and poor oral intake contribute to the energy deficits observed in cancer cachexia. Hormones and mediators, like leptin and serotonin (5-HT), may play role in the development of cancer-induced anorexia.26,27 Leptin reduces appetite and increases energy expenditure via central nervous system.26 Thus, if a disease processes, such as cancer was to produce factors that induce or mimic the hypothalamic effect of excess negative feedback signaling from leptin, the expected outcome would be sustained anorexia (lack of appetite) and cachexia (muscle wasting and uncontrolled weight loss), without the usual compensatory response.28 Increased level of plasma and brain tryptophan, the precursor of 5-HT, and IL-1 may underlie the increased serotonergic activity seen in the cancer cachexia. The 5-HT activates various serotonin receptor subtypes in the gastrointestinal tract and ganglia, exerting a range of biological and physiological effects, such as nausea and vomiting, which can induces anorexia.29 Chemotherapy-related alterations in taste and smell may also contribute to this loss of apetite.30
Myostatin and Insulin-Like Growth Factor-I
Myostatin is an extracellular cytokine that is mostly expressed in skeletal muscles and is known to play a crucial role in the negative regulation of muscle mass.31 Upon binding to the activating type-IIB receptor, myostatin can initiate several different signaling cascades, resulting in decreased muscle growth and differentiation.31 Transgenic mice with the myostatin gene develop a cachexia-like syndrome that manifests with severe wasting.32 On the other side, insulin-like growth factor-I (IGF-1) is highly sensitive to food intake. Under normal conditions, IGF-1 signaling seems to be dominant and blocks the myostatin pathway. However, an inhibition of IGF-1 can occur when myostatin is overexpressed.33 It was shown that in the absence of IGF-1, the level of apoptosis in C2C12 cells treated with myostatin increased,31 and the levels of IGF-1 is reduced in experimental models of cachexia.34
Impact of Cachexia on Cancer
Cachexia has marked effects on QOL, physical function, and mortality in cancer patients when compared with weight-stable patients. One reason for these effects may be related to the increased toxicity related to cancer-directed treatments with body composition changes.35 Drug doses are typically administered on the basis of body surface area, which does not account for muscle loss (i.e., sarcopenia, cachexia), fat gain, or water retention.36 Consequently, the volume of distribution of cancer treatments can be impacted not only from a change in lean body mass, but also from changes in fat mass and total body water. This change in volume of distribution may decrease the effectiveness and/or increase toxicities of cancer directed therapies. Body composition changes as a predictor of toxicity have been documented in breast, lung, esophageal, and colon cancers.37
Cachexia negatively impacts on surgical risk and response to chemotherapy and radiotherapy and ultimately results in decreased QOL.3 Cancer patients experiencing weight loss leading up to and during chemotherapy receive a lower initial dose and experience more frequent and severe dose-limiting toxicity when compared with weight-stable patients,35,37 consequently receiving significantly less treatment. These patients also experienced decreased QOL, performance status and survival intervals and lowered response to treatment.
Screening of Cancer Cachexia
Cachexia screening is performed with the aim of increasing awareness and enabling early recognition and treatment. To detect cachexia at an early stage and to detect its acceleration, regular evaluation of weight change and BMI are needed, beginning at cancer diagnosis and repeated depending on the stability of the clinical situation. Mandatory screening for weight loss in patients with cancer has been established in some countries,38 with the intent of detecting in-hospital malnutrition.
Until now, there are no common assessment tools or validated measurements for screening of cachexia in cancer patients. Due to the lack of a specific cachexia assessment tool, malnutrition assessment tools are used in daily practice (Table 3).39 The most commonly used malnutrition assessment tools are patient-generated subjective global assessment (PG-SGA),40 mini–nutritional assessment (MNA),41 malnutrition-screening tool (MST),42 malnutrition universal screening tool (MUST),43 and nutritional risk screening-2002 (NRS-2002).44 The current malnutrition assessment tools are helpful to screen for malnutrition in health care and these tools are utilized to recommend nutritional support but they do not guide multimodal cachexia therapy. The malnutrition assessment tools only marginally assess the impact of cachexia, whether physical or psychosocial. Some of the instruments include performance status, others ask about depression, but functional impairment caused by cachexia and eating-related distress is not part of the established tools.39
|
PG-SGA |
MNA |
MST |
MUST |
NRS 02 |
|
|---|---|---|---|---|---|
|
Stores depletion |
x |
x |
x |
x |
x |
|
Muscle mass and strength |
(x)a |
xb |
|||
|
Anorexia or reduced food intake |
x |
x |
x |
x |
x |
|
Catabolic drivers |
(x)c |
||||
|
Functional and psychological effects |
x |
x |
Abbreviations: MNA, mini–nutritional assessment; MST, malnutrition-screening tool; MUST, malnutrition universal screening tool; NRS, nutritional risk screening; PG-SGA, patient-generated subjective global assessment.
aOnly physical examination.
bOnly calf circumference.
cOnly fever and corticosteroids.
Diagnosis of Cachexia in Cancer Patient
In the daily routine, very often the diagnosis of cachexia in cancer patients is made on the basis of a reduced food intake. Because of the complex condition of cancer patients, this could be misleading because the reduction of ingested calories might be the consequence of dysphagia or depression rather than a sign of cachexia. The diagnosis of CACS is complex and requires therefore a meticulous clinical examination of the patient (Table 4).45
|
Test |
Finding |
|---|---|
|
Clinical |
|
|
Body weight |
Unintentional weight loss (>5% during preceding 6 months) |
|
Skeletal muscle mass |
Decreases biceps, quadriceps, muscle mass |
|
Food intake recall or diary |
Anorexia and/or decreased food intake |
|
Fatigue |
Increased |
|
Range of motion |
Usually impaired |
|
Quality of life surveys |
Decreased scores |
|
Karnofsky’s Performance Scale |
Decreased scores |
|
Serum |
|
|
Serum C-reactive protein |
Increased (acute-phase response) |
|
Serum fibrinogen |
Increased (acute-phase response) |
|
Serum hematocrit |
Decreased (anemia) |
|
Serum albumin |
Decreased |
Assessment of Weight Loss
The presence of weight loss is an important clinical sign that can even be the first detectable manifestation of the presence of cancer and can be easily obtained by patients and caregivers or measured by health care providers.45 After the possibility of intentional weight loss (for example, by dieting) has been excluded, alternative causes of weight loss of unknown origin are investigated. Weight loss is typically the first element of a cachexia diagnosis, so the presence of unintentional weight loss of more than 5% of premorbid weight in a 6 months period should be assessed.12,46 Weight loss varies in its severity: a 5% loss is considered the threshold of major risk of poor clinical outcome,4,12 with increasing risk as weight loss cumulatively reaches 10, 15, 20%. or higher.47
Assessment of Body Composition
One of the criteria for diagnosing cachexia according to International Consensus is appendicular muscle mass index that consistent with sarcopenia. Because of this, a physical examination has to be performed to evaluate skeletal muscle wasting and loss of body fat. The most important muscles for such an assessment are the gastrocnemius, vastus lateralis, rectus abdominus, and biceps because these type-II fast-twitch muscles are most commonly affected in cancer cachexia.45
Recently, bioelectrical impedance analysis (BIA),48 computed tomography (CT) imaging analysis,49 and dual-energy X-ray absorptiometry (DXA)50 has been introduced as tool to evaluate body composition (Table 5). Bioelectric impedance analysis can also be used to measure body composition based on the electrical properties of tissues so it can estimate body fat percentage, fat mass, fat-free mass, and total body water with the help of predictive equations. BIA, unfortunately, has been reported not to be as reliable as DXA for assessing body composition in cancer patients51; however, BIA can be used to calculate the phase angle which has been reported to predict poor survival in cancer patients.52
|
Measurement |
Tools |
Cut-off value (kg/m2) |
|
|---|---|---|---|
|
Men |
Women |
||
|
Appendicular skeletal muscle mass index |
Bioimpedance analysis |
<7 |
<5.7 |
|
Dual energy X-ray absorptiometry |
<7 |
<5.4 |
|
|
Lumbar skeletal muscle mass index |
Computed tomography scan on L3 |
<46.12 |
<34.18 |
Both DXA and CT imaging both have high precision and specificity for discriminating individual tissue components and are the gold standard for body composition evaluation. DXA uses alternating high-energy and low-energy X-rays to analyze the differences between bone and soft tissue attenuating at different X-ray levels.50 It measure predominantly appendicular muscle. DXA has some limitations including inability to differentiate subsets of adipose tissue into intramuscular, visceral, and subcutaneous and lean body mass into muscle, organ, and tumor, as well as overestimation of lean body mass in settings when changes of >5% hydration status of cancer patients.50
CT is often used over time to monitor cancer and can be taken advantage of to serve as an assessment tool for body composition. CT imaging can discriminate between adipose tissue, bone, organs, and muscle including degree of fatty infiltration by Hounsfield’s units based on tissue-specific attenuation values using software programs including SliceOmatic (TomoVision, Magog, Canada), FatSeg, OsiriX, and ImageJ.53 This method measures body composition through the measurement of muscle tissue located on the level of L3 since it strongly correlates with total body skeletal muscle area.54,55 Limitations of CT imaging include exposure to radiation which can be minimized if CT scans used for standard of care in cancer staging are utilized.
Assessment of Quality of Life and Anorexia
Assessing QOL is critical endpoint in cancer patients with cachexia. The functional assessment of anorexia-cachexia therapy (FAACT) scale consists of the functional assessment of cancer therapy general (FACT-G) scale, and the anorexia–cachexia subscale (ACS) and is a QOL scale specific for cancer patients with cachexia.56 FAACT scale includes five subscales: (1) seven items for physical well-being, (2) six items for emotional well-being, (3) seven items for social well-being, (4) seven items for functional well-being, and (5) 12 items for ACS with each item rated as a five-level scoring system (0–4 points) with a higher sum of all 39-item score equating with a better QOL.56
Another questionnaire has been developed from The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC-QLQ-C30) to measure QOL specifically in cancer patients with cachexia that is called EORTC QLQ-CAX24.57 It contains 24 items which consist of five multi-item scales (food aversion, eating and weight-loss worry, eating difficulties, loss of control, and physical decline) and four single items. This questionnaire can adjunct the EORTC-QLQ-C30 to achieve better measurement of QOL in cancer patients with cachexia.57
Biomarkers
When identifying and monitoring patients with cancer who have cachexia, it is important to acknowledge the potential role of biomarkers. One potential serum biomarker commonly used in clinical practice is C-reactive protein (CRP) which, when combined with additional factors of weight loss and nutritional intake, has identified patients at risk for cancer cachexia.58 Elevated CRP (>10 mg/L) has been linked with weight loss and has been confirmed in numerous studies.59,60 Low serum albumin (<35 g/L) has also been associated with weight loss.61
The modified Glasgow Prognostic Score, which is a combination of albumin and CRP, has been validated and reported to correlate with poor nutritional status and weight loss, decrease response to chemotherapy, and increased sensitivity to toxicities, and is a useful prognostic scoring tool.62,63 Ghrelin, obestatin, and leptin have also been studied as potential biomarker for cachexia. Previous study has reported raised ghrelin serum levels in cancer patients with cachexia, whereas obestatin and leptin concentrations were found to be reduced.64
Treatment
Diet Modification
Provision of adequate nutrition is a mainstay of cachexia treatment, and up to date guidelines for clinical nutrition in oncology are available. The average caloric deficit in weight-losing patients with cancer cachexia is approximately 250 to 400 Kcals/day.65 An average supplementation of 1 calorie/mL has been failed to improve the nutritional status of patients receiving chemotherapy.47,66 The average protein intake in patients with cancer cachexia is approximately 0.7 to 1.0 g/kg per day.66 Food energy intake needs to increase by 300 to 400 kcal per day and protein intake to increase by up to 50% to have an effect on anabolic resistance (recommended intake1.0–1.5 g/kg per day).66 The use of parenteral nutrition in addition to oral nutritional support has been found to result in a short (6–8 weeks) but significant (p < 0.001), prolongation of survival when nutritional goals were achieved according to a randomized trial.67
Exercise
Physical exercise has been suggested as a promising countermeasure for preventing cachexia.68 The rationale for the use of exercise relies on the known dramatic reduction of muscle strength and endurance during cachexia.68 Physical exercise increases insulin sensitivity, protein synthesis rate, and antioxidative enzyme activity.69 It may also lead to suppression of the inflammatory response and an enhancement of immune function.70
There is significant evidence that endurance exercise (e.g., a high number of repetitions performed over extended time periods against relatively low resistance) ameliorates cancer-related fatigue.71 Combination of resistance and aerobic muscle training has been suggested to be incorporated into cachexia treatment programs.71
Pharmacologic Treatment
Corticosteroids
Corticosteroids are widely used as orexigenic agents,72 as they can exert a limited benefit in the management of cancer associated cachexia by improving appetite, caloric intake, pain control, inducing a sensation of wellbeing and reducing nausea. Prednisolone at a dose of 3 × 5 mg and dexamethasone 3 to 6 mg daily has been shown an appetite enhancement respect toplacebo.73 Methylprednisolone given intravenously at a dose of 125 mg daily will ameliorate QOL.74 Nevertheless, these positive effects are of short duration and do not lead to an increase in body weight.75
Progestogens
Megestrol acetate (MA) and medroxyprogesterone acetate (MPA) are synthetic, orally active progestational agents. In several randomized controlled studies, these com pounds have been found to improve appetite, caloric intake, and nutritional status in patients with nonhormone responsive tumors and cancer anorexia–cachexia syndrome.76,77,78 MA has demonstrated a dose-related beneficial effect, in a dose range from 160 to 1,600 mg/day on appetite, caloric intake, body weight gain (mainly fat), and sensation of wellbeing (with an optimal dosage of 800 mg daily).77,78 It is recommended that a patient is started on the lowest dosage (i.e., 160 mg/day) and that the dose is up-titrated according to clinical response.78,79 MPA has similarly been shown to increase appetite and food intake with a stabilization of body weight at a dose of 1,000 mg (i.e., 500 mg twice daily).78,79 Although the drug is safe at doses of 500 to 4,000 mg daily, side effects have been shown to increase above oral doses of 1,000 mg.76
Cannabinoids
Tetrahydrocannabinol (THC) and its derivatives are synthetic pharmaceuticals able to activate cannabinoid receptors and in particular, the CB1 receptors localized in the hypothalamus and the limbic system. Cannabinoids have been investigated in patients with cancer for antiemetic and appetite-stimulant activity.80 Dronabinol is the synthetic oral form of tetrahydrocannabinol (THC), which is the active agent in marijuana thought to be responsible for these effects. The mechanism of action of dronabinol is not completely understood, but its activity is likely mediated by cannabinoid receptor–related processes.81
Ghrelin
Ghrelin is a 28-amino acid peptide hormone mostly produced in the stomach but also in other gastrointestinal tissues. It induces the release of growth hormone from the pituitary gland, stimulates food intake82 and also suppress the production of proinflammatory cytokines.83 In 2007, DeBoer et al observed a significant increase in food intake and weight gain after administration of human ghrelin or a synthetic ghrelin analogous BIM-28131 in a rat model of cancer associated cachexia.84 At present, a phase-II randomized, placebo-controlled, double-blind study, using an oral ghrelin mimetic, demonstrated an improvement in lean body mass, total body mass, and hand-grip strength in cachectic cancer patients.85 Several clinical trials with ghrelin are currently on going.
Thalidomide
TNF-α, IL-6, and IFN-γ have all been implicated in the pathogenesis of cachexia. Thalidomide (a-N-phthalimidoglutarimide) has complex immune-modulatory and anti-inflammatory properties. Thalidomide has been shown to counter TNFα and IL-6 production.86,87 One randomized placebo-controlled trial in patients with cancer cachexia showed that the drug was well-tolerated and effective at attenuating loss of weight and lean body mass in patients with advanced pancreatic cancer.88
Omega-3 Fatty Acids
Eicosapentaenoic acid (EPA) is one of several omega-3 polyunsaturated fatty acids found abundantly in fish oil. Polyunsaturated fatty acids have been proposed to reduce cachexia-associated tissue wasting,89 as well as tumor growth.89,90 EPA downregulates the production of proinflammatory cytokines in both healthy individuals and patients with cancer. Furthermore, the effects of proteolysis inducing factor, a cachectic factor produced by cancer, are also inhibited by EPA.
Conclusion
Patients with cancer are the population at risk to develop cachexia before and after chemotherapy. The loss of weight that happens during chemotherapy will make a poor prognosis. Cachexia can worse chemotherapy toxicity. Cachexia diagnosis needs evaluation of body weight, food intake, and body composition. Dietary modification and physical exercise is the best strategy for cachexia in cancer patients. Some medications that alter appetite and inflammatory cytokines can be added to improve QOL.
Conflict of Interest
None declared.
References
- Pathophysiology of cancer cachexia: current understanding and areas for future research. Cancer Res. 1982;42(02):721s-726s. (suppl)
- [Google Scholar]
- Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(02):90-99.
- [Google Scholar]
- Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006;42(01):31-41.
- [Google Scholar]
- Eastern Cooperative Oncology Group. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med. 1980;69(04):491-497.
- [Google Scholar]
- Prognostic factors of survival in patients with advanced cancer admitted to home care. J Pain Symptom Manage. 2013;45(01):56-62.
- [Google Scholar]
- The prevalence of concern about weight loss and change in eating habits in people with advanced cancer. J Pain Symptom Manage. 2006;32(04):322-331.
- [Google Scholar]
- Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(02):154-159.
- [Google Scholar]
- Cardiac cachexia in early literature: a review of research prior to Medline. Int J Cardiol. 2002;85(01):7-14.
- [Google Scholar]
- Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(05):489-495.
- [Google Scholar]
- The cachexia clinic: from staging to managing nutritional and functional problems in advanced cancer patients. Crit Rev Oncog. 2012;17(03):293-303.
- [Google Scholar]
- Validation of the CAchexia SCOre (CASCO). Staging cancer patients: the use of miniCASCO as a simplified tool. Front Physiol. 2017;8:92.
- [Google Scholar]
- Resting energy expenditure in patients with non-small cell lung cancer. Cancer. 1991;68(07):1616-1621.
- [Google Scholar]
- Resting energy expenditure and body cell mass alterations in noncachectic patients with sarcomas. Surgery. 1987;102(03):465-472.
- [Google Scholar]
- Anticytokine approaches to the treatment of anorexia and cachexia. Semin Oncol. 1998;25(02):53-57. (suppl 6)
- [Google Scholar]
- Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer. 1997;79(09):1828-1839.
- [Google Scholar]
- Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363-2366. (5488)
- [Google Scholar]
- Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(03):370-378.
- [Google Scholar]
- Catabolism of adipose tissue by a tumour-produced lipid-mobilising factor. Int J Cancer. 1999;80(03):444-447.
- [Google Scholar]
- Modulation of adipocyte G-protein expression in cancer cachexia by a lipid-mobilizing factor (LMF) Br J Cancer. 2001;85(05):758-763.
- [Google Scholar]
- Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996-E1006. (5, Pt 1)
- [Google Scholar]
- MG132-mediated inhibition of the ubiquitin-proteasome pathway ameliorates cancer cachexia. J Cancer Res Clin Oncol. 2013;139(07):1105-1115.
- [Google Scholar]
- Leptin and the regulation of body weight in mammals. Nature. 1998;395:763-770. (6704)
- [Google Scholar]
- Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998;92(04):437-440.
- [Google Scholar]
- Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59(18):4493-4501.
- [Google Scholar]
- Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151(08):3773-3782.
- [Google Scholar]
- Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J Clin Oncol. 2009;27(11):1899-1905.
- [Google Scholar]
- The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2(03):143-151.
- [Google Scholar]
- Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486-1488. (5572)
- [Google Scholar]
- Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol. 2009;297(05):C1124-C1132.
- [Google Scholar]
- IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291(03):R674-R683.
- [Google Scholar]
- Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13(11):3264-3268.
- [Google Scholar]
- Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66(04):583-589.
- [Google Scholar]
- Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(09):2107-2118.
- [Google Scholar]
- Prognostic factors in patients with advanced cancer: use of the patient-generated subjective global assessment in survival prediction. J Clin Oncol. 2010;28(28):4376-4383.
- [Google Scholar]
- Mini Nutritional Assessment (MNA) and biochemical markers of cachexia in metastatic lung cancer patients: interrelations and associations with prognosis. Lung Cancer. 2011;74(03):516-520.
- [Google Scholar]
- Comparison of five malnutrition screening tools in one hospital inpatient sample. J Clin Nurs. 2011;20:2144-2152. (15-16)
- [Google Scholar]
- Teaching nutrition integration: MUST screening in cancer. Oncologist. 2011;16(02):239-245.
- [Google Scholar]
- Cancer cachexia syndrome: pathogenesis, diagnosis, and new therapeutic options. Nutr Cancer. 2015;67(01):12-26.
- [Google Scholar]
- Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck. 2007;29(04):401-411.
- [Google Scholar]
- Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67(11):5531-5537.
- [Google Scholar]
- Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(02):95-101.
- [Google Scholar]
- Predictors of discordance in the assessment of skeletal muscle mass between computed tomography and bioimpedance analysis. J Clin Med. 2019;8(03):322.
- [Google Scholar]
- Appendicular skeletal muscle mass reference values and the peak muscle mass to identify sarcopenia among Iranian healthy population. Int J Prev Med. 2018;9:25.
- [Google Scholar]
- Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Appl Physiol Nutr Metab. 2008;33(06):1232-1239.
- [Google Scholar]
- Phase angle for prognostication of survival in patients with advanced cancer: preliminary findings. Cancer. 2014;120(14):2207-2214.
- [Google Scholar]
- A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle. 2017;8(02):285-297.
- [Google Scholar]
- Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(04):1133S-1137S.
- [Google Scholar]
- A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(05):997-1006.
- [Google Scholar]
- The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer. 2016;24(02):661-666.
- [Google Scholar]
- Development of the EORTC QLQ-CAX24, A Questionnaire for Cancer Patients With Cachexia. J Pain Symptom Manage. 2017;53(02):232-242.
- [Google Scholar]
- Biomarkers for cancer cachexia: is there also a genetic component to cachexia? Support Care Cancer. 2008;16(03):229-234.
- [Google Scholar]
- C-reactive protein levels and vitamin d receptor polymorphisms as markers in predicting cachectic syndrome in cancer patients. Mol Diagn Ther. 2012;16(02):115-124.
- [Google Scholar]
- Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med. 2008;46(03):359-364.
- [Google Scholar]
- Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(06):448-454.
- [Google Scholar]
- The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer. 2012;77(02):383-388.
- [Google Scholar]
- Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Support Care Cancer. 2012;20(08):1823-1829.
- [Google Scholar]
- Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med (Berl). 2000;78(10):554-561.
- [Google Scholar]
- Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Curr Treat Options Oncol. 2010;11:107-117. (3-4)
- [Google Scholar]
- Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52(10):1479-1486.
- [Google Scholar]
- Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer. 2004;100(09):1967-1977.
- [Google Scholar]
- Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1(01):9-21.
- [Google Scholar]
- Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical practice guidelines on cancer cachexia in advanced cancer patients. Available at: http://www.cancercachexia.com/literature-watch/43_clinical-practice-guidelines-on-cancer-cachexia-in-advanced-cancer. Accessed May 28, 2020
- Exercise, cachexia, and cancer therapy: a molecular rationale. Nutr Cancer. 2002;42(02):143-157.
- [Google Scholar]
- Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle. 2012;3(02):73-76.
- [Google Scholar]
- Role of corticosteroids in palliative care. J Pain Palliat Care Pharmacother. 2007;21(04):69-76.
- [Google Scholar]
- Methylprednisolone as palliative therapy for female terminal cancer patients. Eur J Cancer Clin Oncol. 1989;25(12):1823-1829.
- [Google Scholar]
- Mechanisms of skeletal muscle degradation and its therapy in cancer cachexia. Histol Histopathol. 2007;22(07):805-814.
- [Google Scholar]
- Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82(13):1127-1132.
- [Google Scholar]
- Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol. 1993;11(04):762-767.
- [Google Scholar]
- Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. 2012;3(03):163-179.
- [Google Scholar]
- Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148(06):3004-3012.
- [Google Scholar]
- A phase II randomized, placebo-controlled, double-blind study of the efficacy and safety of RC-1291 (RC) for the treatment of cancer cachexia. J Clin Oncol. 2007;25:9133.
- [Google Scholar]
- Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54:447-448.
- [Google Scholar]
- Thalidomide for managing cancer cachexia. Cochrane Database Syst Rev. 2012;4(04):CD008664.
- [Google Scholar]
- Mechanism of lipid mobilization associated with cancer cachexia: interaction between the polyunsaturated fatty acid, eicosapentaenoic acid, and inhibitory guanine nucleotide-regulatory protein. Prostaglandins Leukot Essent Fatty Acids. 1993;48(01):105-109.
- [Google Scholar]
- Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103(03):883-891.
- [Google Scholar]
- Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85(21):1743-1747.
- [Google Scholar]