Translate this page into:
Unresected pediatric myoepithelial carcinomas treated with alternating chemotherapy cycles with adjuvant radiotherapy
Address for correspondence: Dr. Michael Dorbad, Department of Anesthesiology, Geisinger Medical Center, 100 North Academy Avenue, Danville, Pennsylvania 17822, USA. mdorbad1@geisinger.edu
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Myoepithelial carcinomas of soft tissue origin are rare tumors in the pediatric population. Due to the rarity of this malignancy, very few cases have been reported in the literature, and a consensus on treatment has not been established. Most myoepithelial carcinomas of soft tissue present in the extremities and are treated with surgical excision followed by adjuvant radiotherapy. We report 2 cases of pediatric myoepithelial carcinoma presenting with vertebral involvement making complete surgical removal impossible. These patients underwent chemotherapy and adjuvant radiotherapy as the main treatment for their primary tumors.
Keywords
Chemotherapy
myoepithelial carcinoma
pediatrics
radiotherapy
rare tumors
Introduction
Myoepithelial carcinoma is a rare tumor typically occurring in the salivary glands.1,2,3 In rare circumstances, it can originate in soft tissue structures in children. Up to 2007, only 29 cases of pediatric myoepithelial carcinoma had been reported.1 From 2005 to 2012, 7 children were registered in the TREP, a national cooperative initiative to improve management of rare pediatric cancers.4,5
Typically, therapy for this tumor is complete surgical excision.1,2,3,4,5,6 However, this is not always feasible. We describe 2 cases of unresectable tumor making chemotherapy and radiotherapy the only viable treatment options available.
Case Reports
Case 1
An 11-year-old previously healthy Caucasian male presented with 3 months history of left posterior shoulder pain. He also had weakness and paresthesia extending to the left thumb and wrist. On physical examination, a cervical mass was noted with atrophy of the left shoulder. He had decreased range of motion on shoulder circumduction and wrist extension with weakness on the hand grip.
Imaging studies revealed a 6.0 cm × 4.5 cm × 6.6 cm well-circumscribed mass extending from C4 to C7 vertebrae with spinal cord compression Figure 1. He underwent left hemi-laminectomy, lymph node resection, and partial removal of epidural tumor for spinal decompression.
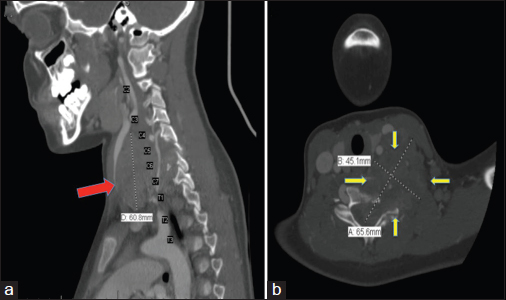
- Computed tomography neck with contrast of Case 1. (a) (Left): Sagittal section showing a heterogeneous infrahyoid neck mass (red arrow) extending from the level of C4 to T1 junction in the supraclavicular region located deep to the sternocleidomastoid muscle and posterior and lateral to the carotid sheath. (b) (Right): Axial section at the level C6-C7 showing an infrahyoid mass (yellow arrows) eroding into the vertebrae. The mass is in direct contact with the upper esophagus, left thyroid lobe, left internal jugular vein, and left common carotid artery
Biopsy showed large atypical, cohesive-appearing cells having eosinophilic cytoplasm with pleomorphic and hyperchromatic nuclei. Immune-peroxidase stains were positive for cytokeratin AE1/AE3, CK7, CK20, Cam 5.2, vimentin, and epithelial membrane antigen (EMA). Cytology was reviewed at Brigham and Women's Hospital, where the biopsy stained positive for PAX8, CK8, CK20, S100, GFAP, AE1/AE3, EMA, Pan-K, INI-1, and SALL4 confirming the diagnosis of myoepithelial carcinoma.
He underwent 8 cycles of intravenous (IV) bleomycin, etoposide, and cisplatin alternating every 3 weeks with carboplatin and paclitaxel. The patient also received a total radiation dose of 5940 cGy in 31 fractions over a 44-day period.
After four months of chemotherapy, positron emission tomography-computed tomography (CT) showed regression of the cervical tumor and lymph nodes. At 8 months, magnetic resonance imaging (MRI) showed a further reduction in tumor size and adenopathy. However, 10 months after initiation of therapy, he developed aggressive pulmonary metastasis and expired thereafter.
Case 2
A 15-year-old Caucasian female presented with 7 months history of low back pain with paresthesias in her bilateral lower extremities. Physical exam was significant only for sacrococcygeal tenderness.
CT with contrast of the chest and pelvis, showed a presacral mass with sclerosis of the sacrum and coccyx with pulmonary nodule suggestive of metastatic disease Figure 2. MRI showed a 4.4 cm × 2.9 cm presacral mass abutting the ventral cortex of the lower sacrum and coccyx, extending into the neural foramina and spinal canal making this tumor unresectable. Thoracotomy with wedge biopsy of the pulmonary nodule was performed.
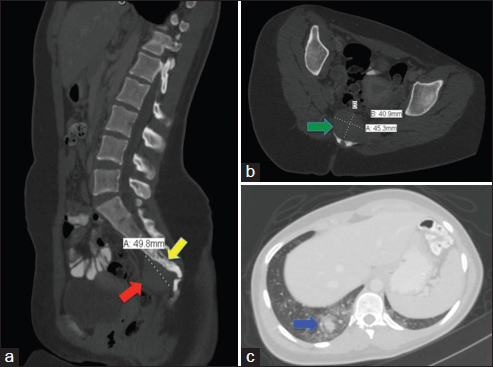
- Computed tomography lumbosacral spine and chest with the contrast of Case 2. (a) (Left): Sagittal section is showing enlarged mesenteric lymph nodes and a 4.3 cm × 3.9 cm × 5.0 cm soft tissue mass (red arrow), inseparable from the lower sacrum and coccyx extending into the spinal canal with sclerosis in the adjacent bones (yellow arrow), (b) (Right upper): Axial section of the sacral mass at the sacrococcygeal level (green arrow), (c) (Right lower): Axial section soft tissue window of the lung showing right lower lobe pulmonary nodule (blue arrow) suggestive of pulmonary metastasis surrounded by ground glass opacity
Lung biopsy showed widespread lymphangitic carcinoma with inflammatory changes. Cytokeratin AE1/AE3, EMA, S-100, vimentin, calretinin, HBME-1, synaptophysin, TTFE-3, and CEA were positive. NUT gene product, GFAP, p63, cytokeratin 7, cytokeratin 20, cytokeratin 5/6, desmin, myogenin, PAX-8, CDX-2, CD99, CD30, CD34, OCT-4, D2-40, chromogranin, CD10, renal cell carcinoma, WT-1, and inhibin were negative. Diagnosis confirmed at Brigham and women's as myoepithelial carcinoma.
She received paclitaxel, carboplatin, and oral etoposide alternating with cycles of IV etoposide and cisplatin. A total of 3000 cGY of radiation was given.
Four months into therapy, surveillance scans showed size regression of the presacral mass and pulmonary nodules. Progressive reduction was noted at 8 months and 14 months following initiation of therapy. She is still alive 42 months from initial presentation.
Discussion
Pediatric myoepithelial carcinomas may be more aggressive than their adult counterpart. The reported mortality rate of pediatric myoepithelial carcinoma is about 43% with a median survival period of 2.5 years.1,4 Complete surgical removal is historically the ideal management for myoepithelial carcinoma.1,2,3,4,5,6 Our 2 cases had unresectable tumors leaving chemotherapy and radiation the only feasible interventions.
Data is limited on chemotherapy for unresectable tumors.1,7,8 An unresectable retroperitoneal tumor with pulmonary nodules was reported by Hornick and Fletcher, where a biopsy was done, but there was no mention of the treatment regimen used.2 Gleason and Fletcher reported 4 patients with unresectable tumors, who nonetheless underwent tumor debulking and excision.1 These patients showed no clinical response and all died within 10 months. The same series mentions 1 successful treatment of metastatic disease several years after surgical removal of the primary tumor, using 5 cycles of doxorubicin and ifosfamide. However, no report exists to show successful use of just chemotherapy and radiotherapy to treat a primary unresectable tumor.
Rosenfeld reported complete response of metastatic disease in a 37-year-old woman treated with carboplatin and paclitaxel.7 Bisogno mentioned complete remission in 6 children treated with ifosfamide, cisplatin, vincristine, and etoposide with radiotherapy.4 Three of the 6 had gross residual disease after incomplete resection. However, none of these children were reported to have unresectable tumor.
There is no standard regimen to treat this malignancy.4,7,9 The combination of cisplatin, etoposide, bleomycin, carboplatin, and paclitaxel was chosen for their proven significant anti-neoplastic effect on mesenchymal, and epithelial tumors in adults and children.3,4,8,10 Oral etoposide was added for the second case to hypothetically induce anti-angiogenesis and radiosensitization without necessarily amplifying toxicity.11 Although Case 2 survived longer than Case 1, it is impossible to conclude if oral etoposide is responsible.
Case 1 survived for 8 months. With the addition of oral etoposide, Case 2 is still alive 42 months from the initial presentation surpassing the recorded median survival time. The regimen was well-tolerated with expected side effects including neutropenia and thrombocytopenia. This prompts us to suggest this regimen as an option for children with unresectable disease.
In summary, we report the results of treating unresectable pediatric myoepithelial carcinoma with alternating cycles of cisplatin, etoposide, bleomycin, carboplatin, and paclitaxel with radiotherapy. To our knowledge, this treatment regimen has never been attempted. We report the first satisfactory result of unresectable pediatric myoepithelial carcinoma with chemotherapy and radiotherapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Myoepithelial carcinoma of soft tissue in children: An aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-24.
- [Google Scholar]
- Myoepithelial tumors of soft tissue: A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-96.
- [Google Scholar]
- Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-1.
- [Google Scholar]
- Myoepithelial carcinoma treatment in children: A report from the TREP project. Pediatr Blood Cancer. 2014;61:643-6.
- [Google Scholar]
- Italian Study on Rare Tumours in Paediatric Age (TREP); Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). The challenge of very rare tumours in childhood: The Italian TREP project. Eur J Cancer. 2007;43:654-9.
- [Google Scholar]
- Epithelial-myoepithelial carcinoma of the lung: A case report and review of the literature. J Pediatr Hematol Oncol. 2009;31:206-8.
- [Google Scholar]
- Lung metastases of epithelial-myoepithelial carcinoma of the parotid gland successfully treated with chemotherapy: A case report. J Oral Maxillofac Surg. 2013;71:220-6.
- [Google Scholar]
- Myoepithelial carcinoma of the breast treated with surgery and chemotherapy. Case Rep Oncol Med. 2013;2013:164761.
- [Google Scholar]
- Effectiveness of carboplatin, etoposide, and bleomycin combination chemotherapy in good-prognosis metastatic testicular nonseminomatous germ cell tumors. J Clin Oncol. 1991;9:62-9.
- [Google Scholar]
- Phase I trial of oral etoposide in combination with radiotherapy in head and neck squamous cell carcinoma – GORTEC 2004-02. Radiat Oncol. 2013;8:40.
- [Google Scholar]







