Translate this page into:
Tumor-associated macrophages: Oblivious confederates in invasive mammary carcinoma
Address for correspondence: Dr. Manoj Gopal Madakshira, Department of Pathology, Armed Forces Medical College, Pune - 411 040, Maharashtra, India. manoj.gopal@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background: The infiltrating margins of carcinomas are associated with presence of inflammatory cell infiltrate which are an integral part of the tumor microenvironment. Amongst the inflammatory cells, Tumor Associated Macrophages (TAMs) play a key role in the tumorigenesis. This study elucidates the density of TAMs in invasive mammary carcinomas and attempts to establish aa association with the following pathological variables: tumor size, histological grade, nodal status, hormonal expression status and Her2Neu overexpression.
Materials and Methods: 90 diagnosed archival cases of invasive mammary carcinomas at a tertiary care centre were included. Density of TAMs was assessed by using CD68 which is a pan-macrophage marker by immunohistochemistry on the archival tissue blocks. The density TAMs (CD68 positive cells) was dichotomised into high (>50 CD68 positive cells/HPF) and low (<5050 CD68 positive cells/HPF) and compared with the above mentioned pathological variables using appropriate statistical tests.
Results: The density of TAMs was significantly higher around the infiltrating edge of the carcinoma in comparison to the adjoining normal terminal duct lobular units. The density of TAMs was more in the infiltrating edge of the tumor than within the tumor nodule/nests. A higher TAM density showed a significant association in tumors having large tumor size, higher histological grade, nodal metastasis, absence of ER and PR expression and Her2Neu overexpression (p value <0.05).
Conclusion: TAMs play an important role in tumor progression in invasive mammary carcinomas. This is as a result of the multiple roles enacted by TAMs in the various stages of tumor development starting from tumor growth, invasion, angiogenesis and metastases. Targeted therapy against TAMs has great potential in the being important components of future treatment strategies against breast carcinomas.
Keywords
Breast
carcinoma
macrophages
Introduction
Tumors are intimately associated with a highly specialized tumor microenvironment.1 The heterogeneous tumor microenvironment has elements of stroma such as fibroblasts and endothelial cells and elements of infiltrating immune cells such as lymphocytes and macrophages Figure 1a.2 The stromal cells and the immune cells contribute to a concoction of growth factors, cytokines, metalloproteinases, and other mediators which orchestrate the development of the tumor.1,3 The tumor microenvironment has been implicated to play an important role in tumor growth regulation, tumor angiogenesis, and metastases.1,2 Of the various immune cells associated with tumor cells, tumor-associated macrophages (TAM) have been shown to comprise the highest proportion Figure 1b.4,5 By the sheer number of macrophages, these cells will have a definite role in tumorigenesis.5,6 Macropahges have been described to have two distinct phenotypes, classical or M1 type and alternate or M2 type.7 The M1 macrophages are formed following exposure to Th1 cytokines, lipopolysaccharides, or endotoxins and are tumoricidal, characterized by the production of nitric oxide synthase-2.7 In contrast, M2 macrophages are formed following exposure to Th2 cytokines and have anti-inflammatory and pro-tumor capacity characterized by the release of transforming growth factor beta, interleukin-4, and interleukin-13.7 It has been shown that most TAMs infiltrating into the tumor are predominantly of the M2 phenotype.3,8
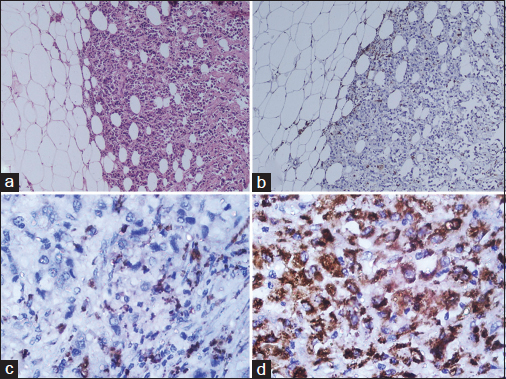
- (a) The invasive front of mammary carcinoma associated with many inflammatory cells (H and E, ×40). (b) CD68 immunohistochemistry (×40) shows the prominent macrophage infiltration around the invasive tumor nests. (c) An example of a low density of CD68 macrophage infiltration (×400). (d) An example of a high density of CD68 macrophage infiltration (×400)
In 2015, more than 100,000 cases of breast carcinoma were diagnosed in India comprising a staggering 10% of all carcinomas diagnosed and lay claim to the dubious distinction of being the most common cancer affecting the female sex.9 Even in the era of triple-testing breast carcinoma continues to be the most important cause of cancer-related mortality.9 Search for newer prognostic and therapeutic markers help in stemming this disease.10 TAMs form an exciting pawn in the realm of tumor immunomodulation which hold promise both as a prognostic marker and a possible target for cancer therapy.7,11,12 This study was based on a hypothesis that an increased TAM density in the invasive front of mammary carcinoma is associated with a higher histological grade and pathological stage.
Material and Methods
The study was undertaken at the Department of Pathology of a tertiary care center from August 2015 to January 2017. Assuming 95% confidence interval, α of 5%, absolute precision of 6%, and based on the literature that about 9% breast carcinoma cases5 have dense infiltration of CD68+ macrophages in tumor stroma, the sample size was calculated to be 87. However, a total of ninety consecutive archival cases were included in our study. The study was cleared by the Institutional Ethics Committee, and approval from the head of department was taken before the use of archival paraffin blocks. Inclusion criteria were all archival cases of invasive mammary carcinoma diagnosed following a radical or breast-conserving mastectomy with axillary lymph node clearance. Exclusion criteria included all cases with a paucity of tumor tissue in the paraffin blocks or lack of archival data.
The sections from archival blocks were reviewed and data on the histological grade, tumor size, nodal status, hormonal receptor status, and human epidermal growth factor receptor 2 (Her2/neu) expression status were tabulated in an excel sheet. The blocks with the invasive front of carcinoma were selected following review. Multiple sequential 4 μ thin sections were taken from the representative blocks. A hematoxylin and eosin stain was done to reassess the histological grade of the tumor as per the modified Scarff-Bloom-Richardson grading.13 Immunohistochemistry using monoclonal CD68 primary antibody (PathnSitu clone KP-1) was performed on the sequential section. A control section of hyperplastic tonsil tissue was run as a control with each batch of immunohistochemistry.14 The density of the pan-macrophage marker (CD68) seen as granular cytoplasmic staining was assessed surrounding the invasive tumor front and stratified as low (<50 CD68 positive cells/hpf) Figure 1c and high (>50 CD68 positive cells/hpf) Figure 1d density. The stratified cases as per CD68 density were then compared with various known prognostic and predictive markers of breast carcinoma such as tumor size, tumor grade, nodal status, hormonal receptor status, and Her2/neu expression. Statistical analysis was done using the Chi-square test in Microsoft Excel version 2010.
Results
A total of ninety archival cases of invasive mammary carcinoma were studied. The presence of TAMs was higher around the infiltrating edge of the tumor in comparison with the tumor cell nests and adjoining normal terminal duct lobular units. A total of 41 cases showed a higher density of TAMs in the invasive front of the carcinoma as against 49 cases which showed a lower density Graph 1. A higher density of TAMs showed a statistically significant association with a larger tumor size (P = 0.010162) Table 1 and Graph 2, higher histological grade (P = 0.014092) Table 1 and Graph 3, nodal metastases (P < 0.00001) Table 1 and Graph 4, absence of ER expression (P = 0.005691) Table 1 and Graph 5, absence of PR expression (P = 0.010488) Table 1 and Graph 6, and Her2/neu overexpression (P = 0.001688) Table 1 and Graph 7.
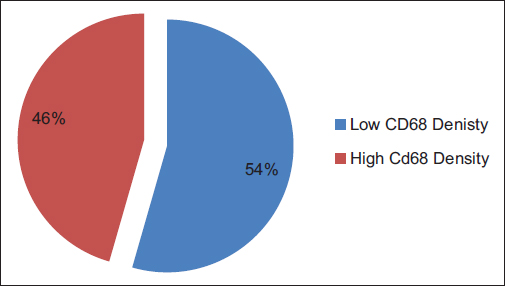
- Graph 1: Dichotomized distribution of CD68 cases as per density
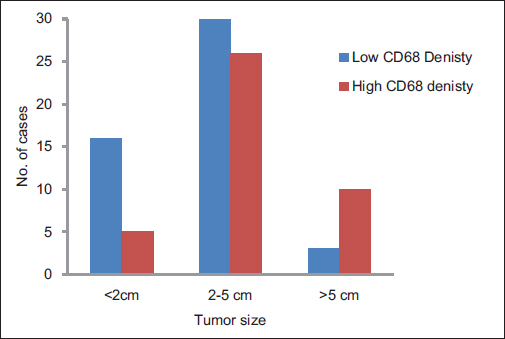
- Graph 2: Distribution of cases as per CD68 density and tumor size
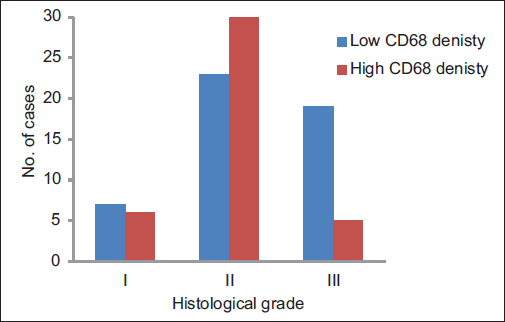
- Graph 3: Distribution of cases as per CD68 density and histological grade
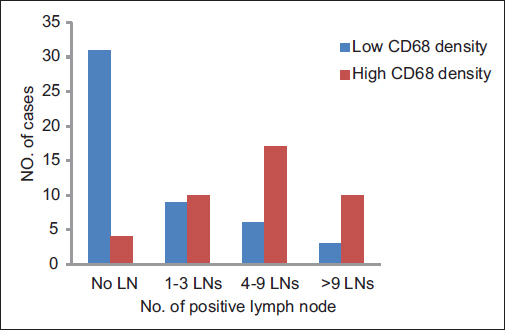
- Graph 4: Distribution of cases as per CD68 density and lymph node status
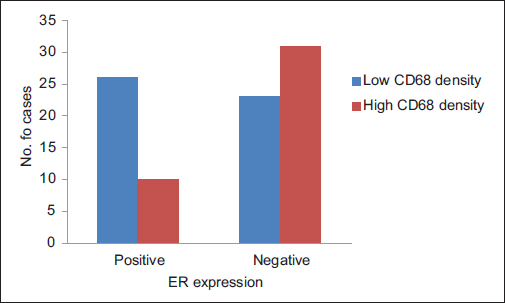
- Graph 5: Distribution of cases as per CD68 density and estrogen receptor expression
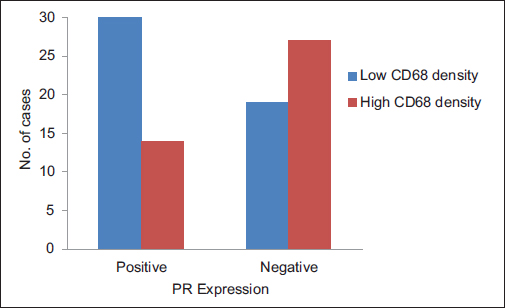
- Graph 6: Distribution of cases as per CD68 density and progesterone receptor expression
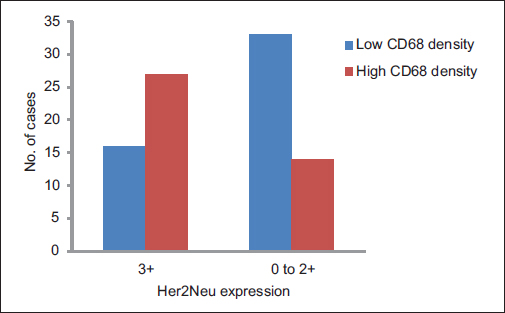
- Graph 7: Distribution of cases as per CD68 density and Her2/neu expression
|
Variable |
Number of cases (%) |
CD68 |
||
|---|---|---|---|---|
|
Low density (%) |
High density (%) |
P |
||
|
Tumor size (cm) |
||||
|
<2 |
21 (23) |
16 (76) |
5 (24) |
0.010162 |
|
2-5 |
56 (63) |
30 (54) |
26 (44) |
|
|
>5 |
13 (14) |
3 (23) |
10 (77) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
|
Histological grade |
||||
|
Grade I |
13 (14) |
7 (54) |
6 (46) |
0.014092 |
|
Grade II |
53 (59) |
23 (43) |
30 (57) |
|
|
Grade III |
24 (27) |
19 (79) |
5 (21) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
|
LN metastases |
||||
|
Absent |
35 (39) |
31 (89) |
4 (11) |
<0.00001 |
|
1-3 LNs |
19 (21) |
9 (47) |
10 (53) |
|
|
4-9 LNs |
23 (26) |
6 (26) |
17 (74) |
|
|
>9 LNs |
13 (14) |
3 (23) |
10 (77) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
|
ER expression |
||||
|
Positive |
36 (40) |
26 (72) |
10 (28) |
0.005691 |
|
Negative |
54 (60) |
23 (43) |
31 (57) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
|
PR expression |
||||
|
Positive |
44 (49) |
30 (68) |
14 (32) |
0.010488 |
|
Negative |
46 (51) |
19 (41) |
27 (59) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
|
Her2/neu expression |
||||
|
3+ |
43 (48) |
16 (37) |
27 (63) |
0.001688 |
|
0-2+ |
47 (52) |
33 (70) |
14 (30) |
|
|
Total |
90 (100) |
49 (54) |
41 (46) |
|
LN - Lymph nodes; ER - Estrogen receptor; PR - Progesterone receptor; Her2 - Human epidermal growth factor receptor 2
Discussion
This study demonstrates the integral part played by TAMs in the tumor microenvironment of breast carcinoma. TAMs have been shown to be intimately associated with the invading front of the tumor in comparison with the adjoining terminal duct lobular units. The higher density of TAM showed a statistically significant association with larger tumor size and higher histological grade similar to earlier studies by Medrek et al. and Zhang et al.5,6 This can be explained by probable presence of protumoral M2 phenotype of TAMs which have been shown to enhance the growth of cancer stem cells by upregulating genes such as SOX-2, OCT-4, Nanong, Sca-1, and AbcG2 in murine models of breast cancer.7,15 These genes are responsible for multiple functions such as enhanced tumorigenicity and resistance to chemotherapy in breast cancer cells.16 Another contributing factor is the role played by TAMs in the inhibition of antitumor immune response.7 Chemical mediators such as prostanoids, prostaglandin E2, transforming growth factor-β, and interleukin-10 released by TAMs have shown to suppress the cytotoxic function of natural killer cells and T lymphocytes.17 TAMs also promote tumor growth by orchestrating the tumor angiogenesis.18 This is explained by the various proangiogenic factors released by TAMs which include vascular endothelial growth factor, epidermal growth factor, platelet-derived growth factor, tumor necrosis factor-α, transforming growth factor-β, interleukin-1 β, interleukin-8, thymidine phosphorylase, and chemokines such as CXCL8 and CCL2.7,19
Higher nodal metastases in breast carcinoma showed a statistically significant association with a higher density of TAMs in the invading front of the tumor comparable to observations concluded by Schoppmann et al. and Chen et al.20,21 This has implications as TAMs have been shown to play a vital role in the metastatic cascade.4 The epithelial–mesenchymal transition of the metastatic clone of tumor cells necessary to initiate the process of invasion has been shown by in vitro studies to be promoted by factors released by TAMs which regulate downstream pathways of β-catenin and E-cadherins.7,22 Studies have also demonstrated the presence of proangiogenic Tie2 positive TAMs in the lymph nodes which have migrated from the tumoral stroma in response to colony-stimulating factor-1 being released by the breast cancer cells. These Tie2-positive TAMs provide the direction for the adventurous clone of cancer cells to reach the axillary lymph nodes.3,7 Epidermal growth factor released by TAMs and colony-stimulating factor-1 released by the adenocarcinoma cells have also been shown to act in tandem to coax the production of podosomes and invadopodia by the TAMs and adenocarcinoma cells which are essential steps for intravasation and degradation of extracellular matrix.23
TAMs exhibited an excellent statistical association with the absence of hormonal receptor status and 3+ expression of Her2/neu. This is in agreement with previous studies which have demonstrated a negative association with luminal type carcinomas. However, no study has been successful in clarifying the reason for selective association of TAMs to a specific subset of the heterogeneous tumor group of breast carcinomas.5,7
Recent research has brought forth the pivotal role of TAMs in breast carcinoma, and many authors have suggested TAMs to be an independent prognostic factor.5 This has led to work into possible therapeutic targets to mitigate the protumorigenic role played by TAMs.10,11,12,24 Three approaches are being studied: impeding recruitment of TAMs to tumor site; selectively destroying TAMs recruited to tumor site, and a possible reprogramming of the TAMs to more desirable antitumoral phenotype. Research into the use of anti-colony-stimulating factor-1, bisphosphonate compounds, and imiquimod have shown promise in this regard and some of the studies are in stage of clinical trials.25,26,27
Conclusion
TAMs are truly the oblivious confederates in breast carcinomas and hold great potential as possible therapeutic targets given their association with specific features of histologic grade, nodal metastases, and nonluminal subtype of breast carcinomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cancer and the tumor microenvironment: A review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571-82.
- [Google Scholar]
- The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-12.
- [Google Scholar]
- Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr Opin Oncol. 2017;29:55-61.
- [Google Scholar]
- Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-6.
- [Google Scholar]
- The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306.
- [Google Scholar]
- High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One. 2013;8:e76147.
- [Google Scholar]
- Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:pii-15025.
- [Google Scholar]
- Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta. 2016;1865:23-34.
- [Google Scholar]
- Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289-95.
- [Google Scholar]
- Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99:180-5.
- [Google Scholar]
- Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000-11.
- [Google Scholar]
- New mechanisms of tumor-associated macrophages on promoting tumor progression: Recent research advances and potential targets for tumor immunotherapy. J Immunol Res. 2016;2016:9720912.
- [Google Scholar]
- Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-upHistopathology. 1991;19:403-10.
- [Google Scholar]
- A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989;73:6-11.
- [Google Scholar]
- Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425-30.
- [Google Scholar]
- Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248-58.
- [Google Scholar]
- Inflammation-associated immune suppression in cancer: The roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38-52.
- [Google Scholar]
- Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064-6.
- [Google Scholar]
- Cancer stem cells and tumor-associated macrophages: A roadmap for multitargeting strategies. Oncogene. 2016;35:671-82.
- [Google Scholar]
- Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947-56.
- [Google Scholar]
- CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541-55.
- [Google Scholar]
- Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res. 2016;76:5302-12.
- [Google Scholar]
- Macrophage-dependent tumor cell transendothelial migration is mediated by notch1/MenaINV-initiated invadopodium formation. Sci Rep. 2016;6:37874.
- [Google Scholar]
- Tumor-associated macrophage-mediated targeted therapy of triple-negative breast cancer. Mol Pharm. 2016;13:1833-42.
- [Google Scholar]
- Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem Soc Trans. 2016;44:333-41.
- [Google Scholar]
- Oral bisphosphonates and improved survival of breast cancer. Clin Cancer Res. 2017;23:1684-9.
- [Google Scholar]
- Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: A Phase 2 clinical trial. JAMA Oncol. 2017;3:969-73.
- [Google Scholar]







