Translate this page into:
Sentinel node biopsy in vulvar cancer: A critical appraisal
Address for correspondence: Dr. Neville F. Hacker, Gynaecological Cancer Centre, Royal Hospital for Women, Locked Bag 1000, Barker Street, Randwick 2031, NSW, Australia. n.hacker@unsw.edu.au
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Since the incorporation of inguinal-femoral lymphadenectomy into the management of patients with vulvar cancer in the mid-20th century, there have been attempts to modify or eliminate the groin dissection to decrease the risk of lower limb lymphedema. Early attempts were significantly flawed and resulted in much unnecessary loss of life because recurrence in an undissected groin is usually fatal. The best compromise yet to decrease the risk of lymphedema is sentinel node biopsy, but accumulated evidence now suggests that the false-negative rate for this procedure, if used for lesions up to 4 cm in diameter, is between 5% and 10%. Most women, properly informed of risks and benefits, are not prepared to take a 1% risk of dying from recurrent vulvar cancer to avoid lymphedema. This is the risk involved, assuming a false-negative rate of 5% and an incidence of positive nodes of 20%. For this reason, sentinel node biopsy should not be considered to be standard practice for patients with early vulvar cancer.
Keywords
Lymphadenectomy
lymphedema
sentinal node biopsy
vulvar cancer
Introduction
The prognosis for patients with vulvar cancer was very poor until the pioneering work of Taussig1 in the United States and Way2 in the United Kingdom in the mid-20th century. By paying careful attention to the dissection of the groin lymph nodes, they were able to improve the survival from 20%–25% to 60%–70% although at the cost of considerable morbidity. This was particularly true after the en bloc approach to radical vulvectomy and bilateral inguinal-femoral lymphadenectomy popularized by Way, after which patients often spent several weeks in hospital healing their groin wounds.
The use of a separate incision approach for the groin dissection slowly became accepted as the standard of care after the 1981 report of 100 patients treated with this approach by Hacker et al.3 In 1990, Micheletti et al. reported that the femoral nodes were located in the fossa ovalis medial to the femoral vein, so there was no need to remove the fascia lata.4 In 1995, Nicklin et al. reported that there could be a 25% reduction in the lateral extent of the groin incision, which helped preserve some lateral lymphatics from the leg which went directly to the axillary nodes.5 These three modifications significantly improved primary groin healing and did not compromise the removal of all groin nodes Figure 1.
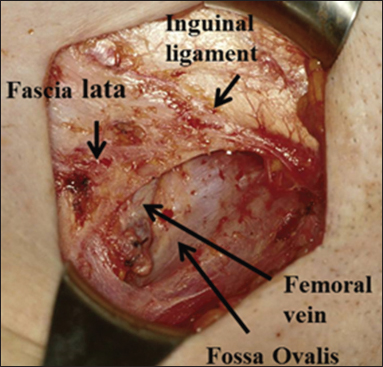
- Inguinal-femoral lymphadenectomy. Note the preservation of the fascia lata and the femoral vein in the fossa ovalis
The status of the groin lymph nodes is the most important prognostic factor for patients with vulvar cancer, but an inevitable consequence of their removal is the later development of lymphedema.6,7 Ryan et al. from our hospital reported lower limb lymphedema in 62% of patients after inguinal-femoral lymphadenectomy.8 Lymphedema is a lifelong affliction, which requires daily attention to massage and support stockings, so it is not surprising that several attempts have been made over many years to reduce or eliminate this risk.
Early Attempts to Decrease Lower Limb Lymphedema
The first attempt to decrease the incidence of lymphedema from groin dissection was from Wharton et al. at the M.D. Anderson Cancer Hospital in Houston in 1974. They defined “microinvasive carcinoma of the vulva” as a lesion ≤2 cm in diameter with ≤5 mm of stromal invasion.9 They reported 25 such patients, none of whom had lymph node metastases, and suggested that lymph node dissection could be omitted from this group of patients.
It soon became apparent that this concept of microinvasion was seriously flawed. Further experience revealed that the only patients at virtually no risk of lymph node metastases were those with a tumor ≤2 cm diameter and with ≤1 mm of stromal invasion. Even patients with 1.1–2 mm invasion had a 7.6% incidence of positive nodes in combined series.10
The second attempt was from DiSaia et al. in 1979. They suggested “superficial inguinal lymphadenectomy” for patients with a lesion ≤1 cm with ≤5 mm stromal invasion. They hypothesized that the superficial inguinal nodes would act as sentinel nodes and that by preserving the femoral nodes, the incidence of lymphedema would be reduced. They reported 18 patients, all of whom had negative nodes, and the survival was 100%.11
It soon became apparent that this approach was also flawed. In 1983, Hacker et al. reported seven patients from four different Cancer Centers in California who recurred in the groin after a superficial inguinal lymphadenectomy.12 Subsequently, the Gynecologic Oncology Group (GOG) in the United States conducted a prospective study of superficial inguinal lymphadenectomy for patients with clinical Stage 1 vulvar cancer, (i.e., ≤2 cm diameter) with ≤5 mm stromal invasion and no clinically suspicious inguinal lymph nodes.13 Once again, the recurrence rate in the groin and subsequent mortality was found to be unacceptably high.
The third attempt to decrease lymphedema involved the use of radiation therapy instead of groin dissection to treat the groin nodes. The GOG conducted a prospective, randomized trial in patients with no clinically suspicious groin nodes. They compared groin irradiation with inguinal-femoral lymphadenectomy (and postoperative radiation for patients with positive nodes) and reported their results in 1992.14 The study was stopped prematurely after only 49 patients had been entered because there was a 19.2% recurrence rate (5 of 26) in the radiation arm versus 0% recurrence rate in the surgical arm (P = 0.02).
With all of these failed attempts to prevent lymphedema, it also became apparent that recurrence in an undissected groin carried a very high mortality. About 90% of these patients were dying of their disease, and this remains true to the present time.10
Lymphatic Mapping
The hypothesis behind lymphatic mapping is that the lymphatic drainage from a tumor occurs in an orderly fashion and will initially go to one or more “sentinel” nodes. If the sentinel node is negative, the remainder of the regional nodes will be negative, so complete lymphadenectomy can be avoided in such patients, thereby decreasing the incidence of lymphedema without compromising survival.
This concept was initially introduced by Cabanas in 1977 for the management of men with penile cancer15 and was later pioneered by Morton et al. in 1992 for the management of melanomas.16
Two complementary techniques have been used to identify the sentinel node(s): The intradermal injection of technitium99 labeled sulfur colloid around the tumor the day before the surgery, and the intradermal injection of a vital blue dye (e.g., isosulfan blue) around the tumor immediately preoperatively.17,18,19 The sentinel node(s) is identified by dissecting the groin and identifying the blue node(s) and by the use of a gamma counter intraoperatively Figure 2. Ultrastaging is undertaken on all negative sentinel nodes using serial sectioning and immunoperoxidase staining for cytokeratin.

- Sentinel node biopsy. (a) Note the blue lymphatics and blue sentinel lymph node, and (b) gamma counter used to identify the radioactive node or nodes
Studies of Sentinel Node Identification in Vulvar Cancer
In 2008, results were published from the multicenter, GROningen International Study on Sentinel nodes in Vulvar cancer study (GROINSS-V).17 It was an observational study, and to be eligible, patients had to have a squamous cell carcinoma of the vulva <4 cm diameter. There were 403 patients recruited to the study, and they underwent 623 sentinel node dissections. Metastatic sentinel nodes were found in 163 groins (26.2%).
Long-term follow-up from this study was reported on 377 patients with unifocal disease in 2016.20 The median follow-up was 105 months. As expected, local recurrence was still a problem, being 24.6% at 5 years and 36.4% at 10 years in sentinel node–negative patients. The isolated groin recurrence rate was 2.5% in sentinel node-negative patients, and all 6 patients died of disease. As expected, short- and long-term morbidity were significantly decreased in patients undergoing sentinel node biopsy.
The median diameter of the vulvar cancers in the long-term follow-up of the GROINSS-V study was only 20 mm (range 3–65 mm), yet the 5-year disease-specific survival was only 93.5%. This is low for such small, node-negative tumors. In 2009, we reported the experience with 121 patients with 2009 International Federation of Gynecology and Obstetrics Stage 1B vulvar cancers of all dimensions, treated at our institution. Five patients (4.1%) underwent radical vulvectomy for multifocality, and the remainder underwent radical local excision. All underwent unilateral or bilateral inguinal-femoral lymphadenectomy and were node negative. With a median follow-up of 84 months, the median overall survival at 5 years was 96.4%.21
Results from other large studies show higher false-negative rates for sentinel nodes.
The results of a multicenter German study were also published in 2008.18 This study enrolled 127 patients with primary T1–T3 vulvar cancer. All patients underwent complete inguinal-femoral lymphadenectomy, and positive nodes were identified in 39 cases (30.7%). Three patients had a false-negative sentinel node, and the authors reported a false-negative rate of 7.7%. However, an additional patient with a midline lesion had a positive sentinel node on one side, but a false-negative node on the other, giving an overall false-negative rate of 10.3%. They concluded that sentinel node biopsy was feasible, but not highly accurate and that the false-negative rate was too high except for T1 (2 cm diameter) tumors. Even with these small tumors, the authors reported a false-negative rate of 6.7%, but the patient with the true positive node on one side and false-negative node on the other side had a primary tumor only 18 mm diameter, so the false-negative rate for T1 tumors was 13.3%.
A Polish study of 56 patients and 109 groin dissections was published in 2010.22 The maximum diameter of the primary tumor was 4 cm, and 99% of patients had both blue dye and lymphoscintigraphy with intraoperative radio localization for sentinel node identification. There were 19 (17%) positive sentinel nodes, but the false-negative rate was 27% (7 cases). The authors concluded: “It is highly probable that the main factor responsible for the high false-negative rate was the surgeon's experience. Although all the operations were performed by surgeons with at least 15 years’ experience, the procedure was performed only a few times by each surgeon.” This is clearly a problem when dealing with an uncommon disease.
In 2010, the GOG published their results on sentinel node biopsy for squamous cell vulvar cancer.19 The study included patients with primary tumors up to 6 cm diameter and no clinically suspicious nodes. In all, 452 patients underwent the planned procedures and 418 (92.5%) had at least one sentinel node identified. At least, a unilateral groin dissection was performed on all patients. There were 132 patients (31.6%) with positive sentinel nodes, including 11 (8.3%) with false-negative nodes. For tumors <4 cm, the false-negative rate was 5.6% (4 of 71).
Long-term follow-up of sentinel node biopsy in patients with vulvar cancer was published from Brown University in 2014.23 They reported results on 69 patients undergoing 111 sentinel node dissections. With a median follow-up of 58.3 months, the groin recurrence rate for patients with negative sentinel nodes was as follows: 0% (0/11) for patients with primary tumors <10 mm, 3.3% (1/30) for tumors 10–20 mm, and 14.3% (2/14) for tumors >20 mm. They concluded that sentinel node dissection was a viable option for patients with squamous cell carcinomas less than 2 cm diameter. However, as we will show below, the majority of patients would not be prepared to accept a 3.3% risk of recurrence and probable death.
In a recent systematic review and meta-analysis of sentinel node biopsy in patients with vulvar cancer, Meads et al. reviewed 29 studies involving 1779 women.24 They reported a false-negative rate of 9% for clinical follow-up of patients with negative sentinel nodes and concluded that this high false-negative rate highlighted the importance of the learning curve effect. However, the learning curve relates more to the detection of sentinel nodes, and only Levenback et al. have looked specifically at this problem. They reported that the failure rate for sentinel node detection was 16% in the first 2 years of their study versus 7% in later years.25
For sentinel node identification in patients with breast cancer, Bass et al. estimated that 23 patients were required by an individual surgeon to achieve a 90% ±4.5% success rate and 53 patients to achieve a 95% ±2.3% success rate.26
Unlike the situation with breast cancer, experience of the individual surgeon with vulvar cancer will always be a problem because the disease is so uncommon.
Recurrence Rate Following Groin Dissection
One of the assertions made by Van der Zee et al. in the GROINSS-V paper was: “The groin recurrence rate in sentinel node-negative patients in the current study (2.3%) seems to be at least comparable to that reported for patients… treated by formal lymphadenectomy of any type.”17
The 2.3% recurrence rate is favorable compared to patients having a superficial inguinal lymphadenectomy, but this technique was discredited by the GOG study of superficial lymphadenectomy previously discussed.13 Robison et al. claimed that the risk of groin recurrence was 5%–7% for patients with disease confined to the vulva having negative nodes after a superficial inguinal lymphadenectomy,23 and Hacker and Eifel, in a literature review, reported a groin recurrence rate of 5.3% (31 of 585 patients) for such patients.10
By contrast, the risk of recurrence in patients having negative nodes after an inguinal-femoral lymphadenectomy is virtually zero. In a literature review, Hacker and Eifel found only 3 groin recurrences out of 780 reported cases (0.4%).10
Quality of Life
Oonk et al. studied the quality of life for patients from the Groningen study after a sentinel node procedure only and compared it to that of patients having an inguinal-femoral lymphadenectomy because of a positive sentinel node.27 The study was performed using the EORTC Quality of Life Questionnaire-Core 36 vulvar-specific questionnaire, and they found no difference in overall quality of life between the two groups, in spite of increased complaints of lymphedema in patients having complete groin dissection. They also compared their results to those of two studies in a healthy population of women over 60 years of age and found that the quality of life for their study population was comparable to that of a general age-matched population. They stated: “Our present study does not support our original idea that a decrease in especially long-term morbidity also translates into an improved quality of life for vulvar cancer patients.”
The critical issue is not about morbidity, predominantly lymphedema – it is about risk. The question that has to be asked is; “What risk of death is a properly informed patient prepared to take, to avoid the risk of lymphedema?”
Farrell et al. undertook a preference study on sixty patients with early vulvar cancer whose treatment at our Institution included at least an ipsilateral inguinal-femoral lymphadenectomy; almost 40% of the patients had lymphedema.28 The patients were asked for their preference between two stated treatment options: Complete groin dissection, which would result in a 60% risk of lymphedema, but a negligible risk of groin recurrence if the lymph nodes were negative, or sentinel node dissection, which would result in a negligible risk of lymphedema, but a 1:100 risk of a groin recurrence, which would usually be fatal. The 1:100 risk was based on a hypothetical false-negative rate of 5% and an incidence of positive groin nodes of 20%.
Given these two choices, 32 patients (53%) said they would take no risk at all with their life, and a further 6 patients (10%) said they would take a 1:1,000,000 risk. Only nine patients (15%) were prepared to take a 1:2 to 1:100 risk, which would be the risk involved if a sentinel node procedure were to be performed Figure 3.

- Women's preference for sentinel node biopsy versus inguinal-femoral lymphadenectomy and the degree of risk each woman would take of missing positive lymph nodes with the sentinel node procedure, modified from Farrell et al28
An earlier study by de Hullu et al. reported similar results on 107 patients previously treated for vulvar cancer with at least an ipsilateral inguinal-femoral lymphadenectomy.29 Sixty percent of patients said they would choose complete lymphadenectomy rather than risk death from the 5% false-negative rate associated with the sentinel node procedure. Interestingly, 60% of 80 gynecologists filling in structured questionnaires were willing to accept a 5%–20% false-negative rate for the sentinel node procedure.
A study from the United States of patient preferences and physician perceptions in the management of breast cancer revealed that women have a strong desire to be involved in the decision-making regarding their treatment, and physicians are unable to consistently predict the treatment decisions that their patients would make.30
Informed Consent
The decision to undertake sentinel node biopsy clearly needs careful discussion of risks and benefits between surgeon and patient. The benefits, particularly the avoidance of lymphedema, would certainly be attractive to the patient, but full disclosure of the risks, namely, a significantly increased likelihood of dying with a groin recurrence, is critical. Many surgeons, unfortunately, take a paternalistic approach, discussing only the benefits, without concern for the risks. This is presumably based on the false assumption that the recurrence rate in the groin will be the same as that following inguinal-femoral lymphadenectomy, and the mistaken belief that the prevention of lymphedema is of paramount importance to the patient.
There is no question that, once acquired, lymphedema is a lifelong affliction requiring daily management by the patient. However, there is also little doubt that as a patient accommodates to her diagnosis of cancer, her mindset changes regarding the type of morbidity, she is prepared to accept to stay alive. In a study of patients with breast cancer, Ganz et al. reported that the cancer experience enriched them, deepened the compassion they felt for others, and changed many of their priorities forever.31
Although the majority of patients are not prepared to take even the slightest risk with their life in return for avoiding lymphedema, some patients are prepared to take the small risk involved. In the senior author's experience, such patients include the frail or the elderly, who fear that they will not be able to manage the support stockings successfully, and younger women whose professional career depends on them having slim legs, such as dancers or models.
Sentinel Node Biopsy in Breast Cancer
In contrast to the situation with vulvar cancer, where sentinel node biopsy is controversial, it is regarded as the standard of care for patients with early breast cancer. In fact, there is now discussion about whether or not it is necessary to undertake complete axillary dissection even in patients with positive sentinel nodes.
Two recent systematic reviews and meta-analyses have evaluated the safety and efficacy of sentinel node dissection alone versus complete axillary lymph node dissection in patients with early breast cancer and sentinel lymph node metastases. A 2013 paper reported three studies with 50,120 patients who had positive sentinel nodes and indicated similar 5-year survival and regional recurrence rates between the two groups of patients.32
A 2015 paper evaluated 12 studies which included 130,575 patients from five randomized controlled trials and seven observational studies – 26,870 patients had undergone sentinel node biopsy alone, while 103,705 had undergone complete axillary node dissection. Although paresthesia and lymphedema were more common in patients having complete axillary node dissection, there were no differences in overall survival (P = 0.35), disease-free survival (P = 0.96), or locoregional recurrence (P = 0.73).33
This excellent outcome is not because the false-negative rate for sentinel node biopsy is any lower in patients with breast cancer than it is for patients with vulvar cancer. In a 2016, systematic review of 24 prospective studies involving 15,462 patients with breast cancer, He et al. reported a pooled false-negative rate of 7.5%.34 The difference is clearly related to the fact that most patients with breast cancer receive adjuvant chemotherapy or hormonal therapy, and respond very well to this treatment, such that axillary nodal recurrence rates are in the order of 0.1%–0.3%.17 There is no effective adjuvant chemotherapy or hormonal therapy available for patients with node-negative vulvar cancer.
In the future, multimodal therapy for breast cancer will be dependent on features in the primary tumor, including molecular markers, potentially rendering the staging information obtained through axillary lymph node dissection inconsequential.35
Conclusions
Sentinel node biopsy is the best strategy yet developed for the virtual elimination of lymphedema in patients with node-negative vulvar cancer, but it should not be regarded as the standard of care. Vulvar cancer is an uncommon tumor, so individual experience is limited, unlike the situation with breast cancer. Nevertheless, from accumulated data, the false-negative rate for both breast and vulvar cancer seems to be between 5% and 10%.
The reason that the axillary node recurrence rate is so low in patients with breast cancer is that most patients will receive adjuvant hormonal or chemotherapy, which is presumably effective against microscopic nodal metastases. By contrast, there is no effective adjuvant therapy for patients with vulvar cancer, so patients with false-negative sentinel nodes will recur in the groin and usually die of their disease.
Although the risk of groin recurrence and death is very low, the majority of patients properly informed about risks and benefits are not prepared to take this risk. In a study at our own institution, about 80% of patients reported that they would rather take a 60% risk of developing lymphedema than a 1% risk of dying of a groin recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cancer of the vulva: An analysis of 155 cases (1911-1940) Am J Obstet Gynecol. 1940;40:764-79.
- [Google Scholar]
- Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet Gynecol. 1981;58:574-9.
- [Google Scholar]
- Deep femoral lymphadenectomy with preservation of the fascia lata. Preliminary report on 42 invasive vulvar carcinomasJ Reprod Med. 1990;35:1130-3.
- [Google Scholar]
- An anatomical study of inguinal lymph node topography and clinical implications for the surgical management of vulval cancer. Int J Gynecol Cancer. 1995;5:128-33.
- [Google Scholar]
- Management of regional lymph nodes and their prognostic influence in vulvar cancer. Obstet Gynecol. 1983;61:408-12.
- [Google Scholar]
- Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study) Am J Obstet Gynecol. 1991;164:997-1003.
- [Google Scholar]
- Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust N Z J Obstet Gynaecol. 2003;43:148-51.
- [Google Scholar]
- Vulvar cancer In: Berek J S, Hacker N F, eds. Gynecologic Oncology (6th ed.). Philidelphia: Wolters Kluwer; 2015. p. :560-607. editors.
- [Google Scholar]
- An alternate approach to early cancer of the vulva. Am J Obstet Gynecol. 1979;133:825-32.
- [Google Scholar]
- Superficially invasive vulvar cancer with nodal metastases. Gynecol Oncol. 1983;15:65-77.
- [Google Scholar]
- Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: A prospective study of the Gynecologic Oncology Group. Obstet Gynecol. 1992;79:490-7.
- [Google Scholar]
- Groin dissection versus groin radiation in carcinoma of the vulva: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1992;24:389-96.
- [Google Scholar]
- Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-9.
- [Google Scholar]
- Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884-9.
- [Google Scholar]
- German Multicenter Study Group. Validation of the accuracy of the sentinel lymph node procedure in patients with vulvar cancer: Results of a multicenter study in GermanyGynecol Oncol. 2008;111:282-8.
- [Google Scholar]
- Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: A gynecologic oncology group study. J Clin Oncol. 2012;30:3786-91.
- [Google Scholar]
- Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol. 2016;140:8-14.
- [Google Scholar]
- Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet Gynecol. 2009;113:895-901.
- [Google Scholar]
- The accuracy of the sentinel lymph node concept in early stage squamous cell vulvar carcinoma. Gynecol Oncol. 2010;116:473-7.
- [Google Scholar]
- Long-term follow-up of vulvar cancer patients evaluated with sentinel lymph node biopsy alone. Gynecol Oncol. 2014;133:416-20.
- [Google Scholar]
- Sentinel lymph node biopsy in vulval cancer: Systematic review and meta-analysis. Br J Cancer. 2014;110:2837-46.
- [Google Scholar]
- Intraoperative lymphatic mapping and sentinel node identification with blue dye in patients with vulvar cancer. Gynecol Oncol. 2001;83:276-81.
- [Google Scholar]
- The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg. 1999;189:183-94.
- [Google Scholar]
- A comparison of quality of life between vulvar cancer patients after sentinel lymph node procedure only and inguinofemoral lymphadenectomy. Gynecol Oncol. 2009;113:301-5.
- [Google Scholar]
- Quality of life after complete lymphadenectomy for vulvar cancer: Do women prefer sentinel lymph node biopsy? Int J Gynecol Cancer. 2014;24:813-9.
- [Google Scholar]
- What doctors and patients think about false-negative sentinel lymph nodes in vulvar cancer. J Psychosom Obstet Gynaecol. 2001;22:199-203.
- [Google Scholar]
- Treatment decisions for breast carcinoma: Patient preferences and physician perceptions. Cancer. 2002;94:2076-80.
- [Google Scholar]
- Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183-99.
- [Google Scholar]
- Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: A systematic review and meta-analysis. Eur J Cancer. 2013;49:812-25.
- [Google Scholar]
- Axillary lymph node dissection versus sentinel lymph node biopsy alone for early breast cancer with sentinel node metastasis: A meta-analysis. Eur J Surg Oncol. 2015;41:958-66.
- [Google Scholar]
- The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: A systematic review. BMC Cancer. 2016;16:107.
- [Google Scholar]
- Axillary node interventions in breast cancer: A systematic review. JAMA. 2013;310:1385-94.
- [Google Scholar]







