Translate this page into:
Robotic neck surgery: Rationales and evolutions
Address for correspondence: Prof. Yoon Woo Koh, Department of Otorhinolaryngology, Yonsei University College of Medicine, Severance Hospital, Yonsei University Health System, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Republic of Korea. ywkohent@yuhs.ac
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Conventional operations for various surgical neck lesions adopted the transcervical scar, which were disfiguring and caused various postoperative morbidities. The advent of the surgical robotics as a result of advancement in technology led to a technical breakthrough in the field of head and neck surgery. Together with the application of the robot, we have seen the promising role of the retroauricular (RA) approach from its versatile applications. This review will discuss in detail various robotic head and neck surgeries via RA approach.
Keywords
Minimally invasive surgery
neck surgery
retroauricular approach
robot
Introduction
Conventional operations for various neck pathologies, which need surgical removal, were accomplished with the adoption of a transcervical scar. This in most cases resulted in a grossly noticeable scar. Considering the fact that the neck is the most easily recognized and exposed area, this unfavorable postoperative cosmesis may be a particular burden for many patients. In conventional neck dissections for head and neck malignancies, not only will the operated patient be displeased from the large surgical scar, but he/she will suffer from various associated postoperative morbidities such as fibrotic band formation and cervical lymphedema. The prolonged postoperative recovery and functional deterioration would pose a detrimental impact to the patient.
Consequently in order to reduce the extent of surgical trauma and minimize these surgery-related morbidities, numerous surgical approaches from a distant port have been developed. These so-called “remote access surgeries” were founded upon the technological advances of endoscopy and surgical robotics. Til date, the main routes for remote access surgeries of the neck are transoral (TO), transaxillary (TA), and retroauricular (RA) approach. Previous reports on the attempts of endoscopic neck surgery via TO route have been limited to resection of submandibular gland (SMG), benign anterior neck mass, and thyroglossal duct cyst[1,2,3,4,5] Recently, the surgical robotics has been exploited with the TO approach in removing the thyroid gland and the entire larynx.[6,7,8,9,10,11] Perhaps, the most widely acknowledged and popularized surgical approach would be the TA approach. The gasless TA approach surgery was initially reported by Dutta et al.,[12] but its full-scale application with the incorporation of the robot was achieved by Kang Sw et al. who have practiced the largest number of robotic TA thyroidectomies.[13,14] Based on the early attempts of robotic facelift thyroidectomies by Terris et al.[15,16,17,18,19] and the authors’ extensive surgical experience on endoscopic and robotic gasless TA thyroidectomy,[20,21] we have extrapolated the application of the RA approach to nearly all aspects of head and neck surgery with the aid of the robotic system. The authors have seen the promising role of RA approach from its versatile applications. This review will discuss in detail various robotic head and neck surgeries via RA approach.
Advantages and Disadvantages of Robotic Neck Surgery via Retroauricular Approach
The advantages of RA robotic neck surgery mainly arise from the fact that the surgical access is made from a distant site, rather than the utilization of the robot. By avoiding the transcervical scar, the RA robotic surgery will offer the better cosmetic outcome and produce significantly lower rate of surgical morbidity. In cases of robot-assisted neck dissection (RAND), the operated patient will be less likely suffering from the postoperative neck wound problems such as wound dehiscence, fibrotic band formation, and cervical lymphedema, considering the fact that in conventional neck dissections, which require an extensive area of dissection, wound dehiscence around trifurcation points and wound problems following postoperative radiotherapy may occasionally occur. These certain difficulties may be diminished in minimally invasive surgeries, yet another set of complications due to the gas insufflation may specifically arise, not to say that the evident transcervical scar cannot be totally avoided, whether how small it may be. What's more comparing with other remote access surgeries such as TA approach, the area of dissection is much less and the operating head and neck surgeon will be more familiar to the local anatomy since the area of dissection is similar to the conventional transcervical neck surgery. Unlike the TA approach, any chances of intraoperative complications due to the surgical procedures related to the obstructing clavicle such as great vessel injury and esophageal perforation will be completely eliminated from the RA approach.
This is a cosmetically excellent procedure, but there are few drawbacks. First, the overall operation time would be longer than conventional methods, mainly due to the additional skin flap elevation and working space creation. Second, this specific surgical technique requires a considerable level of technical proficiency so it would be not a comfortable procedure for a novice endoscopic surgeon. Finally, the total medical cost from this robotic surgery would be naturally more costly than the conventional neck surgery.
Preoperative Considerations
Patient
The patient’ the patient Considerations be naturally mo, length, and circumference of the neck may influence the surgical process. However through our extensive surgical experience of RA robotic neck surgery, obese patients with relatively long necks may prolong the total operation time, but these are not an absolute contraindication for this procedure. Although there was no cases where the surgical procedure was converted to open surgery, the possibility of such situation must always be explained before operation.
Robotic surgical system
All RA robotic procedures described in this article are conducted using the da Vinci S/Si robotic system (Intuitive Surgical Inc., Sunnyvale, CA). Generally, a 12 mm 30° face down dual endoscope is placed at the center of the RA port and two instrument arms (choice of 5 mm Maryland forceps, 5 mm Harmonic Curved Shears, 5 mm Spatula monopolar cautery, or 8 mm ProGrasp forceps) are placed at either side. Recently, the advent of the da Vinci Xi system with enhanced three-dimensional surgical imaging and improved surgical robotic instruments has allowed the placement of an extra 4th robotic arm in the RA port. This has greatly improved the surgical dissection at the robotic console, together with the enhanced optical view.
Surgical Settings
Skin incision
The RA incision is similar to the modified facelift (MFL) incision when performing conventional parotidectomy, except that there is the absence of the preauricular limb [Figure 1]. The robotic surgery is performed exclusively through the RA approach, however, adopting the MFL approach may be considered if a parotidectomy is conducted at the same time or if the surgeon feels that an extra working space is more comfortable.

- Position of the patient and design of the skin incision of the retroauricular incision
Position of patient
The patient is positioned supine with the head rotated to the contralateral side of the approach. The neck is usually relaxed in its natural position without the need for any extension. It is helpful if the ipsilateral auricle is deflected away and maintained with Ioban™ taping.
Surgical sequence
After appropriate patient positioning, the RA incision is made and a working space is established first. Then certain surgical steps of gross dissection under the naked eye are conducted beforehand, to move on to the robotic dissection. When the da Vinci Xi system is utilized for the operation though, the initial steps of the surgery can be performed directly at the robotic console, since the deployment of an extra robotic arm greatly facilitates the process.
Operative Techniques
Robotic surgery of benign neck mass
Remote access surgery for benign neck mass has been more appealing since the postoperative cosmesis is of greatest concern due to the benign nature of lesion. Since the initial reports of Dutta et al.,[12] there have been several attempts of endoscopically removing the benign neck lesions via TA[22,23,24] or RA approach.[25] Although solitary lesion locating near the clavicle or the superior mediastinum can be sufficiently removed using the TA approach,[26] nearly all benign mass can be comfortably removed through the RA approach alone.[27,28]
Robotic excision of neurogenic tumor
The skin flap is elevated underneath the platysma muscle and an adequate area of working space is established before the placement of a self-retaining retractor. The robotic arms are then docked. For the removal of neurogenic tumors, a Metzenbaum scissors (PKissors (PKogenic tumors a Metzenbaum scissors (PKre pl is done to expose the contour of the neurogenic tumor. Using the dissecting forceps and scissors, the true capsule of the neurogenic tumor is revealed and the tumor is enucleated to minimize the postoperative neural damage[29] [Figure 2].
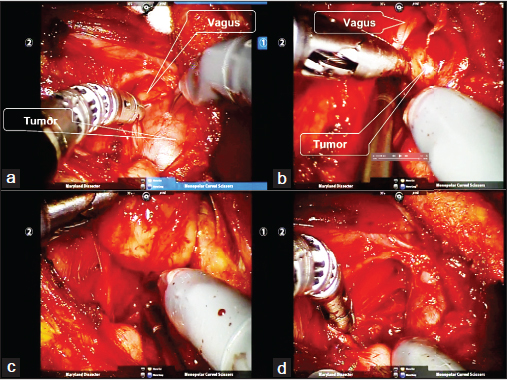
- Robot-assisted excision of the neurogenic tumor via a retroauricular approach. (a) Identification of the tumor capsule and the vagus nerve. (b and c) Subcapsular dissection of the tumor. (d) Note that enucleation of the tumor was accomplished with preserving the nerve sheath of the vagus
Robotic Sistrunk's operation
After subplatysmal skin flap elevation through the RA incision, the outline of the cystic lesion can be usually easily identified beneath the strap muscles. After robotic arms docking, the robotic dissection is initiated at the midline of the neck by dividing the fibroadipose tissue at the anterior neck using a 5 mm Maryland forceps and a 5 mm spatula monopolar cautery. The cystic lesion is carefully dissected and mobilized and the hyoid bone is identified and skeletonized in continuation with the tumor. Once the ipsilateral side of the hyoid great horn is sufficiently mobilized, a conventional type bone cutter is directly introduced through the RA port by the patient-side assistant surgeon and is cut. Further dissection is done to the contralateral side and the remaining hyoid bone is cut as also with the bone cutter. Further the extension of the thyroglossal duct is followed beyond the hyoid bone and finally the main mass together with the resected hyoid bone is removed en bloc through the RA port[30] [Figure 3].

- Robot-assisted Sistrunk operation. (a) After resecting the left great horn of the hyoid bone, infrahyoid muscles together with fibroconnective tissue is dissected and freed away from the tumor using DaVinci Si system. (b) After identifying the right great horn of the hyoid bone, the tumor is carefully dissected from the surrounding tissue and mobilized using DaVinci Xi system
Robotic submandibular gland resection
The previous reports of SMG resection via remote access ports located in the RA region[31,32,33] have formed the basis of our RA robotic SMG resection. After appropriate positioning of the patient and placing the RA incision, sufficient amount of working space is established, and next the robotic arms are docked. Once the robotic arms are placed, the contour of the SMG is identified and the dissection is commenced by opening the fascia at the lower border of the SMG. Subcapsular dissection is continued with Harmonic curved shears or monopolar cautery until the proximal portion of the facial artery is identified which is controlled either by Harmonic curved shears or vascular clips. Retracting the SMG posteriorly, the anterior aspect of the SMG is exposed. The SMG ganglion and Wharton's duct are ligated with the preservation of the lingual nerve and hypoglossal nerve [Figure 4]. The RA robotic SMG resection procedure is the basic surgical procedure for any type of RAND, so it is advised for a novice robotic surgeon to sufficiently conduct this surgery before attempting RAND.
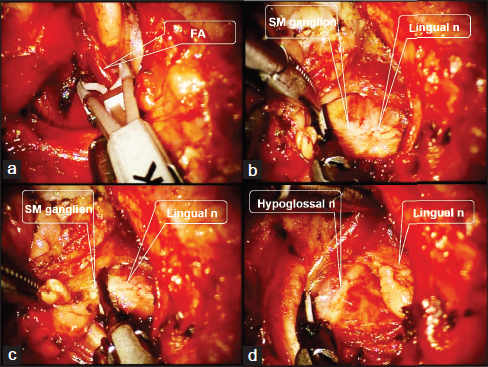
- Operative procedures of robotic submandibular gland excision. (a) The facial artery is ligated with Hem-o-lok Ligation System (Teleflex Inc., Research Triangle Park, NC) during robotic submandibular gland excision of the left side. (b and C) The submandibular ganglion is ligated using the Harmonic scalpel during robotic submandibular gland excision of the left side. (d) The submandibular gland of the left side is removed and the hypoglossal nerve and the lingual nerve are preserved
Robotic neck surgery in head and neck cancer
Since the first surgical experience of RAND in 2010, our institution has actively engaged in practicing RAND and has steadily reported its feasibility for both cN0 and cN+ necks.[34,35,36,37,38,39,40,41,42] Furthermore, free flap reconstruction can be competently performed through the RA port.[43,44]
Robotic supraomohyoid neck dissection
Our institution has previously reported the feasibility of RAND of levels I-III in oral cavity cancer, SMG cancer, and parotid cancer.[36,37,38,39,40] Specific details of the surgical procedures are described herein.
Prerobotic step [Figure 5]

- Distal facial artery and marginal mandibular branch of the facial nerve. The marginal branch of the facial nerve should be identified and preserved
Using the da Vinci S/Si system, certain “preparatory” surgical steps must be conducted beforehand, before applying the robotic arms. However, using the da Vinci Xi system, the robotic arms are introduced once the working space has been established and the following steps can be directly performed at the robotic console.
After establishing the sufficient amount of working space, the following steps are performed under direct vision. The procedures are concordant to the conventional techniques of neck dissection except that the approach is made posteriorly. The initial dissection is made by identifying the marginal branch of the facial nerve. After level Ia dissection, level IIb is dissected following the skeletonization of the spinal accessory nerve (SAN). Then levels IIa and III are dissected with the specimen directing toward the carotid sheath.
Robotic step [Figure 6]
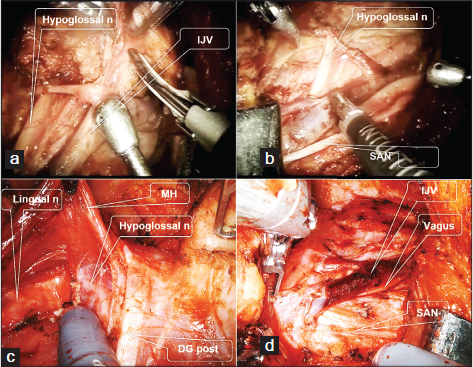
- Level I-III dissection of the right side with DaVinci Si and Xi system. (a) Dissection of lymphoadipose tissues from the carotid sheath and internal jugular vein using DaVinci Si system. (b) Surgical field after SND level I-III using DaVinci Si system. (c) Lingual nerve and hypoglossal nerve should be safely preserved after level Ib dissection using DaVinci Xi system. (d) Surgical field after SND level I-III using DaVinci Xi system. MH - Mylohyoid muscle, DG post - Posterior belly of digastric muscle, SAN - Spinal accessory nerve SND - Selective neck dissection
The robotic dissection step is started at level Ia and is continued down to the predissected specimen of levels Ib and II. The remaining dissection is accomplished at level III around the carotid sheath. The specimen is then removed en bloc, and careful examination of the final surgical field is done.
Robotic comprehensive neck dissection (levels I-V or II-V)
On the basis of the successful experiences of RAND for cN0 necks, the authors have carefully extended the indications to cN+ necks.[41,42] The main purpose of RAND is to minimize the postoperative morbidities, so the SAN, internal jugular vein (IJV), and the sternocleidomastoid (SCM) muscle should all be preserved in every case. Any neck metastases with extensive invasions which require inevitable removal of any of these structures are discouraged in performing RAND. Therapeutic RAND should therefore be performed under strict indications where there are no gross, extensive invasions of the metastatic node according to close examinations of preoperative imagings.
Prerobotic step [Figure 7]

- Levels IIB, VA, IIA, and upper III dissections. With the upward retraction of the skeletonized SCM muscle, levels IIB, VA, and the lateral aspect to the carotid sheath of IIA, and upper III are dissected under direct vision or under the console work with DaVinci Xi system. Branches of the spinal accessary nerve should be identified and preserved. SCM - Sternocleidomastoid muscle, SAN - Spinal accessory nerve
Again, once the working space has been created, this step may be directly done at the robotic console if the da Vinci Xi system is utilized.
If level I is included in the procedure, the gross dissection is commenced by identifying the marginal branch of facial nerve as described earlier for the supraomohyoid neck dissection. When performing the neck dissection of levels II-V, the dissection is started at level II with the visualization of the inferior border of the SMG. Opening up the fascia at the inferior margin of SMG, the dissection is continued posteriorly and the SAN is identified. After skeletonizing, the entire course of SAN, the posterior fascia of SCM muscle is opened and the muscle is lifted upward with a retractor. Levels IIb and Va are dissected and the specimen is retracted medially toward levels IIa and upper III. After completion of levels IIa and upper portion of III dissection, the specimen is primarily removed for maintaining optimal surgical exposure. The self-retaining retractor is readjusted to retract the SCM muscle together with the skin flap.
Robotic step [Figure 8]

- Stepwise procedures of modified radical neck dissection (level I-V) with DaVinci Si system. (a) Level I dissection. Lingual nerve and hypoglossal nerve should be safely preserved after level Ib dissection. (b) Carotid sheath dissection. The fibrofatty tissues near carotid sheath in level IV is dissected using Harmonic Curved Shears. (c) Identification and preservation of the vagus nerve. The transverse cervical artery is verified and the phrenic nerve is located beneath the transverse cervical vessels. (d) Ligation of the lymphatic duct. The lymphaticduct is sealed with Hem-o-lok to prevent a chyle leakage. MH - Mylohyoid muscle, LNs - Lymph nodes, SCM - Sternocleidomastoid muscle, IJV - Internal jugular vein
If level I dissection is required, robotic dissection is done at level Ia. It would be helpful if the level I specimen is removed before moving on to levels IV and V dissection. The previously dissected tissue of level Va is held with the robot and dissection is carried out in a superior to inferior manner. As the dissection progresses to level Vb, the specimen is retracted medially where it meets the omohyoid muscle. The transverse cervical artery and the phrenic nerve running underneath the vessel should be visualized and preserved during the dissection at this level. The dissection is continued medially toward level IV until it meets the carotid sheath. During this step, the lymphatic or thoracic duct is routinely ligated using hemoclips or Hem-o-lok ligation system to prevent the possibility of future chyle leakage. Branches of IJV can also be ligated with harmonic curved shears or Hem-o-lok ligation system during carotid sheath dissection. The final neck specimen is delivered through the RA port and the surgical field is carefully inspected for any bleeding or any damage to major neurovascular structures.
Robotic thyroid surgery in thyroid tumors
Since authors have developed for the 1st time in the world and actively performed RAND via MFL or RA approach in various head and neck cancers in 2010 and based on our increasing surgical experiences,[34,35,36,37,38,39,40,41,42] we could safely adopt this particular technique to robotic thyroidectomy including the total thyroidectomy and also simultaneous modified radical neck dissection through the same RA port where needed.[45,46,47,48]
Prerobotic step [Figure 9]

- Robotic Hemi-thyroidectomy using DaVinci Xi system. (a) The omohyoid muscle and the strap muscles are identified under the console works. (b) The omohyoid muscle and the sternothyroid muscle are delineated from the thyroid gland. (c) The working space is easily obtained with three robotic instruments. (d) The omohyoid and sternothyroid muscles are retracted superiorly and secured by the 3rd robotic arm. The anterior border of the sternocleidomastoid muscle can be retracted posteriorly
Again, using the da Vinci S/Si system, certain “preparatory” surgical steps can be conducted, before applying the robotic arms. However, using the da Vinci Xi system, the robotic arms are introduced once the working space has been established and the following steps can be directly performed at the robotic console.
Creating a sufficient amount of working space is the key to successful operation. Therefore an adequate area of skin flap elevation is mandatory. After skin incision along the predesigned line, flap elevation is continued in a subplatysmal plane and is carried out anteriorly to the midline of the neck to reveal the contralateral lobe of thyroid gland and superiorly to the inferior border of the mandible and inferiorly toward the sternal notch. The first step of dissection is delineating the anterior border of the SCM muscle, which is then retracted posteriorly to expose the carotid sheath. The contour of the thyroid gland is brought into view and the omohyoid muscle is identified. The omohyoid muscle and strap muscles underneath are all dissected and held upwards by the retractor and secured to reveal the superior pole of the thyroid gland.
Robotic step [Figure 10]

- Robotic Hemi-thyroidectomy using DaVinci Xi system. (a) The superior thyroid artery and vein are ligated with fenestrated bipolar forceps to mobilize the superior pole of the ipsilateral thyroid gland. (b) Following the release of the superior pole of the thyroid gland, thyroid isthmusectomy and dissection of pyramidal lobe are executed to expose the anterior tracheal wall. (c) The thyroid gland is then retracted upwards and medially and the recurrent laryngeal nerve can be identified near the Berryr the Berryte (d) Surgical field after the right heme-thyroidectomy with the ipsilateral central compartment neck dissection
The da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, CA) is introduced via the RA port with a face down 30° dual-channel endoscopic camera arm at the center, and two robotic instrument arms placed at either side each mounted with 5 mm Maryland forceps and Harmonic curved shears. After robotic arms docking, the robotic procedure begins by dissecting at the previously exposed superior pole of ipsilateral thyroid lobe. Using the Harmonic curved shears, the superior thyroid vessels are identified and ligated. Next the superior pole of thyroid gland is grasped and lifted off the trachea toward the lower pole. During this process of careful dissection along the thyroid capsule, the parathyroid glands are easily noted at the posterior surface of the gland where it is reflected off and preserved. At the medial aspect of the superior pole, the isthmus of thyroid gland is identified and consequently, thyroid isthmusectomy is executed using Harmonic curved shears. As the dissection proceeds toward the inferior pole, the recurrent laryngeal nerve (RLN) can be soon visualized along the lateral border of the thyroid gland. The dissection is then done away from the RLN and continued downward to remove the ipsilateral lymph nodes of the central compartment. All efforts should be made not to damage the RLN and the parathyroid glands, especially when handling Harmonic shears and suction tips. Next the specimen is reflected superiorly and medially and the dissection is continued medially to meet the previously dissected thyroid isthmus. Finally, the rest of the ipsilateral thyroid lobe is dissected away from the trachea and the connecting Berry's ligament and the specimen is removed after freeing the final attachments of the ipsilateral central compartment lymph nodes.
In cases of total thyroidectomy, the instrument arm holding the Maryland dissector forceps is changed to ProGrasp forceps for contralateral thyroidectomy. The 30° face down endoscope is still used for visualization but the orientation of the robotic arms is realigned to direct more medially. Also, the patient's bed is tilted approximately 15-30° to the ipsilateral side (ipsilateral downwards, contralateral upwards). Dissection for contralateral thyroidectomy is started at the remaining thyroid isthmus. Dissection is continued superiorly toward the upper lobe of the contralateral thyroid gland and the superior thyroid artery and vein are each found and ligated, which will enable the complete mobilization of the superior pole. The thyroid gland is held up and pushed away and the dissection is conducted in a medial to lateral direction. The lymphoadipose tissue of the paratracheal and paraesophageal lymph nodes is dissected. As the dissection progresses, the contour of the contralateral RLN is visualized. Taking care not to damage the nerve, dissection is done away from the RLN and at the same time, the nerve is traced along inferiorly toward the central compartment. The retrieved specimen is directed superiorly and together with the rest of the thyroid gland, the specimen is dissected and released from its surrounding attachments and finally taken out.
Postoperative Considerations
Postoperative care
The postoperative recovery course is no different to conventional neck surgery. Generally, a closed suction drain is inserted behind the hairline and placed in the surgical field. It is monitored for postoperative drainage. Potential postoperative complications are similar to the conventional neck surgery. There are certain specific complications with relation to the RA robotic surgery, which needs special attention. The risk of direct or indirect injury to the marginal mandibular nerve may be relatively increased so special caution must be paid when elevating the RA flap around the mandible and retracting tissue to expose level I. Risks of postoperative bleeding, hematoma formation, or chyle leakage are not particularly increased with the RA robotic surgery however, if any of these situations develop during the postoperative course, it is likely that the situation must be controlled by approaching from the RA port under general anesthesia. Therefore, it is important to thoroughly examine the final surgical field before closing and make preventive measures if needed. Skin discoloration or ischemic changes may develop at the RA site, but most can be resolved conservatively. There have been incidences of hair loss along the hairline incision so it is advised to bevel the cut when making the incision along the hairline.
Surgical outcomes
According to our previous comparative studies of RAND with the conventional operation,[40,42] patients who have received the robotic surgery were significantly more satisfied with their cosmetic outcomes. Also, perioperative parameters such as intraoperative blood loss, amount and duration of postoperative drainage, hospitalization period, number of retrieved lymph nodes, and complication rates were comparable to the conventional neck dissection. However, the total operation time was significantly longer for the RAND. Similar results were noted from other institutions.[49,50,51] The greatest advantage from RA robotic thyroid surgery would be an excellent cosmetic outcome [Figure 11]. In comparison with conventional open methods using a transcervical incision, an obvious cervical scar would be completely eliminated by placing the surgical incision behind the auricle and within the hairline. Additionally, when compared with the TA thyroidectomy, the thyroid gland, and lymph node tissues at the neck would be more easily reached due to the decreased area of dissection. Also, the central compartment lymph nodes would be easier to address and the risk of intraoperative brachial plexus injury or any other physical sequelae resulting from the specific positioning of the patient for TA approach would be diminished.

- Postoperative photo of patients who received robotic retroauricular hemi-thyroidectomy with ipsilateral central compartment neck dissection
Future Directions
The most concerning drawback of the RA robotic surgery was that the operation has been conducted within a relatively limited working space. Even if a sufficient amount of working space has been created, there was hardly any extra space once all three robotic arms are docked. This was the case when using the da Vinci S/Si system, but as previously described, the utilization of the Xi system has been most beneficial, since an additional 4th robotic arm can be placed through the RA port. Considering the progress of surgical technology, if a flexible, multi-instrument mounted, single robotic arm is to be developed and commercially available, it would facilitate even more the RA robotic neck surgery. Not only would the RA robotic surgery be easier to learn and practice, but the surgical skill itself could be further refined by placing a smaller incision.
Conclusion
From the universal application of the RA approach together with the robot, the paradigm shift of the transcervical incision to the RA incision in neck surgery will open a new era of head and neck surgery. Combining RAND with TO robotic surgery, the patient will be provided with a truly minimally invasive head and neck surgery. Furthermore, its clinical application is expected to be promising. The authors have novel yet limited surgical experiences of performing hypopharyngeal tumor excision via lateral pharyngotomy through the same RA port, after completing RAND. Total glossectomy with free flap reconstruction has been successfully accomplished through the RA approach (unpublished data). Further evaluation of its surgical outcomes to verify the feasibility and oncologic safety will be required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Transoral excision of the submandibular gland. Otolaryngol Head Neck Surg. 2007;137:343-5.
- [Google Scholar]
- Intraoral removal of the submandibular gland: A new surgical approach. Otolaryngol Head Neck Surg. 2000;122:798-802.
- [Google Scholar]
- Intraoral removal of a thyroglossal duct cyst using a frenotomy incision. Thyroid. 2011;21:1381-4.
- [Google Scholar]
- Endoscope-assisted intraoral resection of external dermoid cyst. Head Neck. 2012;34:907-10.
- [Google Scholar]
- Transoral robotic total laryngectomy: Report of 3 cases. Head Neck. 2013;35:E338-42.
- [Google Scholar]
- Robotic transoral periosteal thyroidectomy (TOPOT): Experience in two cadavers. J Laparoendosc Adv Surg Tech A. 2015;25:139-42.
- [Google Scholar]
- Transoral robotic-assisted thyroidectomy with central neck dissection: Preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg. 2011;5:279-282.
- [Google Scholar]
- Transoral periosteal thyroidectomy: Cadaver to human. Surg Endosc. 2015;29:898-904.
- [Google Scholar]
- “Stealth surgery”: Transaxillary subcutaneous endoscopic excision of benign neck lesions. J Pediatr Surg. 2008;43:2070-4.
- [Google Scholar]
- Robot-assisted endoscopic surgery for thyroid cancer: Experience with the first 100 patients. Surg Endosc. 2009;23:2399-406.
- [Google Scholar]
- Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: The operative outcomes of 338 consecutive patients. Surgery. 2009;146:1048-55.
- [Google Scholar]
- Robotic facelift thyroidectomy: I. Preclinical simulation and morphometric assessmentLaryngoscope. 2011;121:1631-5.
- [Google Scholar]
- Robotic facelift thyroidectomy: II. Clinical feasibility and safetyLaryngoscope. 2011;121:1636-41.
- [Google Scholar]
- Robotic facelift thyroidectomy: Patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech. 2011;21:237-42.
- [Google Scholar]
- Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg. 2012;147:20-5.
- [Google Scholar]
- Robotic facelift thyroidectomy: Facilitating remote access surgery. Head Neck. 2012;34:746-7.
- [Google Scholar]
- Endoscopic hemithyroidectomy with prophylactic ipsilateral central neck dissection via an unilateral axillo-breast approach without gas insufflation for unilateral micropapillary thyroid carcinoma: Preliminary report. Surg Endosc. 2010;24:188-97.
- [Google Scholar]
- Endoscopic thyroidectomy via a unilateral axillo-breast approach without gas insufflation for unilateral benign thyroid lesions. Surg Endosc. 2009;23:2053-60.
- [Google Scholar]
- Endoscopic removal of a cystic neck mass via an axillo-breast approach. Laryngoscope. 2011;121:571-3.
- [Google Scholar]
- Endoscopic axillo-breast approach for benign neck mass excision. Laryngoscope. 2012;122:559-64.
- [Google Scholar]
- Endoscopic excision of branchial cleft cyst in the neck using mammary areolae and axilla approach: A case report. Surg Laparosc Endosc Percutan Tech. 2012;22:e284-7.
- [Google Scholar]
- Endoscopic resection of upper neck masses via retroauricular approach is feasible with excellent cosmetic outcomes. J Oral Maxillofac Surg. 2013;71:520-7.
- [Google Scholar]
- Robot-assisted resection of multiple schwannomas of the neck and mediastinum through an axillary approach. Artif Organs. 2012;36:647-8.
- [Google Scholar]
- Robotic resection of benign neck masses via a retroauricular approach. J Laparoendosc Adv Surg Tech A. 2013;23:578-83.
- [Google Scholar]
- Robot-assisted excision of branchial cleft cysts using a postauricular facelift approach. Auris Nasus Laryn×. 2015;42:424-7.
- [Google Scholar]
- Robot-assisted Sistrunk operation via a retroauricular approach for thyroglossal duct cyst. Head Neck. 2014;36:456-8.
- [Google Scholar]
- Removal of the submandibular gland by a retroauricular approach. Arch Otolaryngol Head Neck Surg. 2006;132:783-7.
- [Google Scholar]
- Endoscopic resection of the submandibular gland via a hairline incision: A new surgical approach. Laryngoscope. 2010;120:970-4.
- [Google Scholar]
- Endoscope assisted submandibular sialadenectomy: The face-lift approach. Eur Arch Otorhinolaryngol. 2011;268:619-22.
- [Google Scholar]
- Gasless transaxillary robot-assisted neck dissection: A preclinical feasibility study in four cadavers. Yonsei Med J. 2012;53:193-7.
- [Google Scholar]
- Feasibility of robot-assisted neck dissections via a transaxillary and retroauricular (“TARA”) approach in head and neck cancer: Preliminary results. Ann Surg Oncol. 2012;19:1009-17.
- [Google Scholar]
- Robot-assisted selective neck dissection via modified face-lift approach for early oral tongue cancer: A video demonstration. Ann Surg Oncol. 2012;19:1334-5.
- [Google Scholar]
- Robotically assisted selective neck dissection in parotid gland cancer: Preliminary report. Laryngoscope. 2013;123:646-50.
- [Google Scholar]
- Robot-assisted selective neck dissection combined with facelift parotidectomy in parotid cancer. Head Neck. 2014;36:592-5.
- [Google Scholar]
- Robotic-assisted neck dissection in submandibular gland cancer: Preliminary report. J Oral Maxillofac Surg. 2013;71:1450-7.
- [Google Scholar]
- Robot-assisted Supraomohyoid neck dissection via a modified face-lift or retroauricular approach in early-stage cN0 squamous cell carcinoma of the oral cavity: A comparative study with conventional technique. Ann Surg Oncol. 2012;19:3871-8.
- [Google Scholar]
- Robot-assisted selective neck dissection of levels II to V via a modified facelift or retroauricular approach. Otolaryngol Head Neck Surg. 2013;148:778-85.
- [Google Scholar]
- Therapeutic robot-assisted neck dissection via a retroauricular or modified facelift approach in head and neck cancer: A comparative study with conventional transcervical neck dissection. Head Neck. 2015;37:249-54.
- [Google Scholar]
- Free flap reconstruction after robot-assisted neck dissection via a modified face-lift or retroauricular approach. Ann Surg Oncol. 2013;20:891-8.
- [Google Scholar]
- Robot-assisted free flap in head and neck reconstruction. Arch Plast Surg. 2013;40:353-8.
- [Google Scholar]
- Endoscopic retroauricular thyroidectomy: Preliminary results. Surg Endosc. [Epub 15/04/2015 A head of Print]
- [Google Scholar]
- Robotic excision of a huge parathyroid adenoma via a retroauricular approach. J Craniofac Surg. 2015;26:e55-8.
- [Google Scholar]
- Robotic total thyroidectomy with modified radical neck dissection via unilateral retroauricular approach. Ann Surg Oncol. 2014;21:3872-5.
- [Google Scholar]
- Robot-assisted neck dissection via a transaxillary and retroauricular approach versus a conventional transcervical approach in papillary thyroid cancer with cervical lymph node metastases. J Laparoendosc Adv Surg Tech A. 2014;24:367-72.
- [Google Scholar]
- Robotic selective neck dissection by a postauricular facelift approach: Comparison with conventional neck dissection. Otolaryngol Head Neck Surg. 2014;150:394-400.
- [Google Scholar]
- Robot-assisted level II-IV neck dissection through a modified facelift incision: Initial North American experience. Int J Med Robot. 2014;10:391-6.
- [Google Scholar]







