Translate this page into:
Quality of Life among Survivors of Locally Advanced Cervical Cancer Treated with Definitive Chemoradiotherapy in a Decade of Transition
Address for correspondence Warren Bacorro, MD, Department of Clinical Epidemiology, University of Santo Tomas, Faculty of Medicine and Surgery, España Boulevard, Sampaloc, Manila, 1015 Metro Manila, Philippines. wrbacorro@ust.edu.ph
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction The standard treatment for locally advanced cervical cancer (LACC) is concurrent chemoradiotherapy (CRT). External beam radiotherapy (EBRT) and brachytherapy (BRT) advances in the last decade have resulted in improved local control and survival. There is a lack of data on quality of life (QoL) among survivors.
Objective This systematic review aimed to synthesize published data on QoL among LACC survivors treated with CRT and determine clinical factors of QoL.
Methods Systematic literature search was conducted in PubMed, EBSCO, and ScienceDirect for relevant articles published in 2010 to 2020. Eligible studies on LACC survivors aged 18 years and above, who reported QoL after CRT, were included. Screening and data extraction were done by two pairs of independent reviewers.
Results Five cohort studies, three cross sectional studies, and one clinical trial were included. Reported temporal evolution of QoL varied: two studies reported improvement of overall QoL, while four reported worsening of symptoms. Gastrointestinal, genitourinary, sexual, and psychosocial domains showed significant impairment. Age, stage, and baseline distress and physical condition were clinical determinants of body image, sexual activity, menopausal symptoms, distress, and dyspnea. Peripheral neuropathy, lymphedema, and dyspnea were reported, while grade 3 to 4 gastrointestinal, genitourinary, and musculoskeletal toxicities were rare.
Conclusion Use of advanced EBRT and BRT techniques is associated with improving QoL in the first 3 years from treatment completion. Gastrointestinal, genitourinary, sexual, and psychosocial functions remain impaired on the long-term. Other late toxicities worth noting include peripheral neuropathy, lower limb edema, and insufficiency fractures.
Keywords
cervical cancer
cancer survivors
quality of life
chemoradiotherapy
late toxicities
Introduction
Cervical cancer is the fourth most common cancer in women.1 In 2018, around 570,000 women were diagnosed to have cervical cancer, and almost half died of this disease.2 Despite worldwide implementation of prevention and early detection strategies, like the Papanicolaou smear and Human Papillomavirus vaccination, cervical cancer remains to be prevalent and is associated with significant morbidity, especially among the low to middle income countries. It has a crude annual incidence rate of 13.6 per 100,000 women, with up to 30% of new cases being diagnosed at a locally advanced stage (IIA to IVA).3
The current standard treatment for locally advanced cervical cancer (LACC) is concurrent chemoradiation (CRT). As demonstrated by five landmark trials, the addition of cisplatin-based chemotherapy to external beam radiotherapy (EBRT) improved oncologic outcome for LACC.4,5,6,7,8 A meta-analysis in 2008 of 18 randomized trials showed a 6% improvement in the 5-year survival with CRT compared with EBRT alone.9 In the past decade, advancements in EBRT and brachytherapy (BRT) led to improvement in locoregional control and toxicity. These include the use of three-dimensional conformal (3DCRT) and intensity-modulated radiotherapy (IMRT),10 nodal boost,11,12 image-guided radiotherapy,13 image-guided brachytherapy (IGBT),14 and hybrid intracavitary-interstitial brachytherapy (ICISBT) techniques.15 These strategies are aimed to improve tumor coverage and increase tumor dose, while improving organ-sparing, thus improving local control and decreasing radiotherapy-related complications.
Cervical cancer will affect quality of life (QoL) from diagnosis, during treatment, and up to the survivorship phase. This can be positive or negative and will differ at different time points.16 As treatments improve survival, more emphasis should be given to QoL and survivorship. Since late toxicities could be persistent and their impact on QoL pervasive,16,17 preventative, supportive, rehabilitative, and compensatory strategies need to be defined. While reasons for looking into these long-term QoL outcomes are compelling, most of the randomized controlled trials with radiotherapy have a limited follow-up period.18 There is a lack of data regarding long-term QoL and treatment-related side effects among LACC survivors.
This systematic review synthesized published data from 2010 to 2020 on long-term QoL and toxicity among LACC survivors treated with definitive CRT (CRT followed by BRT). Furthermore, the investigators sought to review clinical determinants of QoL.
Methodology
Study Design
This systematic review protocol was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses Protocols (PRISMA-P) 2015 statement. Literature search was checked according to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews (PRISMA-S). Clinical trials, cohort, cross-sectional, and case–control studies were eligible for this research. Case reports, case series, qualitative studies, news articles, textbook chapters, and reports or summaries from conferences were excluded due to their study design. Only articles written in English were included.
Search Strategy and Data Sources
A comprehensive literature search was performed in PubMed (including MEDLINE, PubMed Central, and Bookshelf) and EBSCO (including Biomedical Reference Collection: Basic, CINAHL Plus with Full Text, eBook Collection, MEDLINE Complete, and Psychology and Behavioral Sciences Collection). A MEDLINE search strategy was made via PubMed then adapted for other databases (Supplementary Material 1, available in the online version only). Search terms used were keywords related to cervical cancer, CRT, and QoL. Specific details on search strategies used in this study were outlined (Supplementary Material 2, available in the online version only). Searches were limited to studies published from 2010 to 2020.
ScienceDirect was searched independently on their website. To conduct the manual search, three strategies were used: reference screening, citation tracking, and related articles checking. References of systematic reviews which were accepted for full text screening were also checked. Citation tracking and related articles checking were performed via Google Scholar. Unclear data on the included papers was further sought by emailing the authors for clarifications.
Participants and Study Outcomes
The population being investigated was women with LACC of squamous, adenocarcinoma, or adenosquamous histology, who have undergone definitive CRT, and the primary outcome was QoL, measured using validated questionnaires administered at ≥1 year from completion of treatment. Definitive CRT is the concurrent administration of chemotherapy and EBRT to the pelvic region, with inclusion of the para-aortic and/or inguinal regions as indicated, followed by BRT, with primary curative intent. This does not include CRT given before (preoperative/neoadjuvant) or after (postoperative/adjuvant) surgery, or CRT given after neoadjuvant chemotherapy. Articles were included as long as they had reported on the above-specified population and outcome, even if a subset did not, given that the data could be extracted.
Study Selection
Search results from all databases were checked for duplicates using the Mendeley Reference Manager and then it was checked manually. Initial screening for eligibility was done using the titles and abstracts. Full texts were then retrieved and assessed independently for final eligibility by any two reviewers (V.H.C., P.A.C., R.J.C., R.M.C., Y.N.C.). Any disagreement was resolved by consensus (V.H.C., P.A.C., R.J.C., R.M.C., Y.N.C., K.K.Y., W.B.).
The following articles were excluded: studies that reported only women with precancerous cervical lesions, with cancers other than cervical cancer, with histology other than specified above (neuroendocrine, small cell, sarcoma, lymphoma), or who underwent forms of treatment other than definitive CRT; studies that do not report on QoL at ≥1 year from the completion of treatment; and studies not in the English language, with unavailable full-text or are abstract-only, and with incomplete data.
Data Extraction and Analysis
Two pairs of reviewers (P.A.C. and R.J.C., R.M.C. and Y.N.C.) independently extracted data, and a third (V.H.C., K.K.Y., W.B.) reviewer reviewed the extracted data for completeness and accuracy. Disagreements were resolved by consensus. Information extracted from each included study was based on: (1) age of study participants; (2) stage of disease; (3) inclusion and exclusion criteria of the study; (4) EBRT technique: 3DCRT or IMRT, versus two-dimensional (2D) conventional; (5) BRT technique: IGBT versus 2D; (6) use of ICISBT; (7) concurrent chemotherapy agent/s; (8) QoL tool and timing of administration; and (9) severity and nature of late toxicities. The International Federation of Gynecology and Obstetrics (FIGO) staging used during the treatment of the patients or as indicated in the publication was noted as is. The extracted data were analyzed and synthesized qualitatively.
Critical Appraisal and Risk of Bias
Any two independent reviewers (V.H.C., P.A.C., R.J.C., R.M.C., Y.N.C.) performed critical appraisal and risk of bias assessment using the NIH tool for observational studies and controlled intervention studies. Disagreements were resolved by consensus. The response (yes, no, cannot determine/CD, not reported/NR, not applicable/NA) to each item in the applicable appraisal checklist and the rationale were noted. Considering all responses, each article was deemed to be with low, high, or unclear risk for bias.
Results
Search Results
The search was conducted on October 15, 2020. Fig. 1 shows the flow diagram for selection of studies.
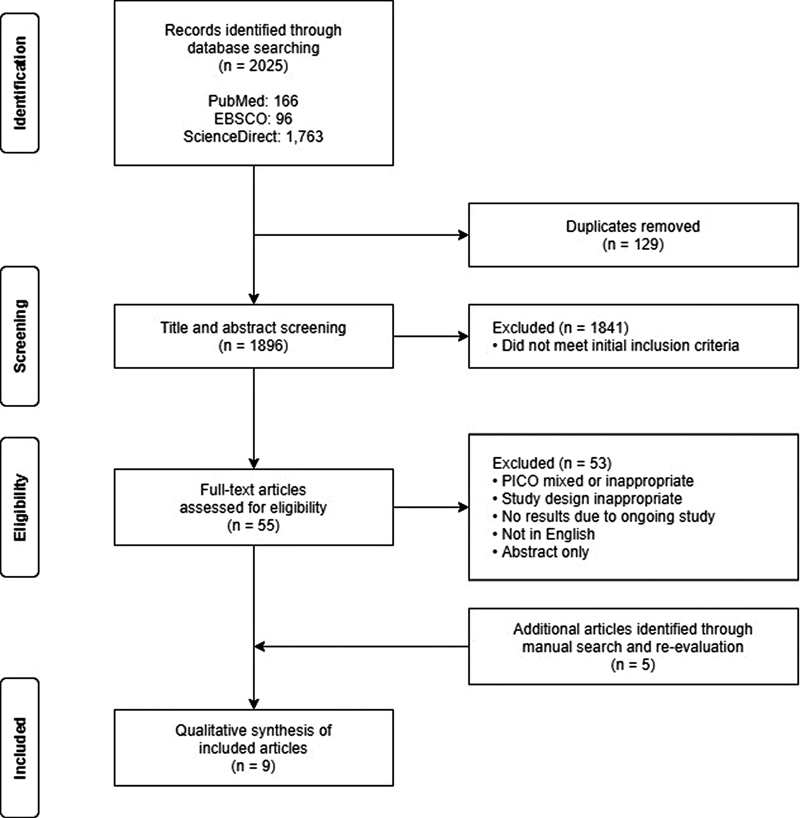
-
Fig. 1 Flow diagram for selection of studies.
A total of 2,025 articles were identified by searching the databases, of which 129 duplicates were removed. All remaining articles underwent title and abstract screening, and 1,841 articles were excluded after not meeting the initial inclusion criteria. From these, 55 articles remained, and subsequent full-text assessment yielded four eligible papers.
Manual search was performed after, wherein 1,215 studies were retrieved and screened. Of these, nine papers were retrieved for review. Four were excluded, as they were identified to be updated, outcome-specific analyses of an already included study. Five were retained, making a total of nine studies for inclusion in the final review and synthesis.
Study Characteristics
The characteristics of the included papers are shown in Table 1. Of the included articles, five were cohort studies, three were cross-sectional studies, and one was a phase II randomized clinical trial. The studies were conducted in Asia (four), Europe (two), and America (two); one was multinational.
|
Study and year published |
Type of study |
Country |
Number of patients |
Participant eligibility |
QoL tool |
Timing of administration (n) |
|
|---|---|---|---|---|---|---|---|
|
Inclusion criteria |
Exclusion criteria |
||||||
|
Clinical trial |
|||||||
|
Nunes de Arruda et al 2020 |
Phase II RCT |
Brazil |
107 52, CRT 55, NACT + CRT |
● Cervical cancer ● FIGO IB2-IVAa ● ≥1 QoL evaluation |
● Disease recurrenceb ● Did not complete QoL questionnaire ● ECOG > 2 ● Peripheral neuropathy > 2 |
EORTC QLQ-C30 EORTC QLQ-CX24 |
3 mo 6 mo 9 mo 12 mo (36) |
|
Cohort |
|||||||
|
Conway et al, 2020 |
Retrospective |
Canada |
67 |
● Cervical cancer ● FIGO IB-IVA ● MRI-guided IGBT ● Age >18 ● QoL at baseline (before or within 14 d of start of EBRT) and ≥1 follow-up |
● Not statedc |
ESAS-r |
24 mo; 15–45 (Median; range) |
|
Gargiulo et al, 2016 |
Prospective |
Italy |
59 25, CRT 34, NACT + RH |
● Cervical cancer ● FIGO IB2-IVA ● Complete response lasting ≥18 mo |
● Not statedc |
EORTC QLQ-C30 EORTC QLQ-CX24 IIQ-7 |
36 mo |
|
Kirchheiner et al, 2016 |
Prospective |
Multinationald |
744 |
● Cervical cancer ● FIGO IB-IVA ● CRT and IGBT |
● Disease recurrencec |
EORTC QLQ-C30 EORTC QLQ-CX24 |
12 mo (474) 24 mo (295) 36 mo (190) |
|
Rai et al, 2014 |
Prospective |
India |
35 |
● Cervical cancer ● FIGO IB2-IIIB ● MRI-guided IGBT |
EORTC QLQ-CX24 |
12 mo |
|
|
Ljuca and Marošević 2011 |
Prospectivef |
Bosnia and Herzegovina |
35 |
● Cervical cancer ● FIGO IIB ● CRT |
● Other FIGO stages except IIB ● Non-CRT treatment |
EORTC QLQ-CX24g |
12 mo |
|
Cross-sectional |
|||||||
|
Daga et al, 2017 |
Cross-sectional |
India |
48 |
● Cervical cancer ● FIGO IIB-IVA ● Age >18 ● Completed CRT ≥2 y prior to study entry |
● Disease recurrence ● Mental illness or cognitive impairment |
FSFI |
≥2 y |
|
Prasongvej et al, 2017 |
Cross-sectional |
Thailand |
43 |
● Cervical cancer ● FIGO I-IV ● Age 30–70 ● Treated from 1996 to 2015 ● No evidence of other malignancies |
● Language barrier ● Severe medical condition ● Severe psychological disease ● Use of illegal drugs ● Nursing home residence |
EORTC QLQ-C30 (Thai Version) |
4.1 y ± 3.8 (Mean ± SD) |
|
Katepratoom et al, 2014 |
Cross-sectional |
Thailand |
35 |
● Cervical cancer ● Completed CRT before June 2008 |
● Disease recurrence ● Incomplete concurrent CRT ● Adjuvant RT after RH ● Urinary tract infection, urinary obstruction, vesicovaginal fistula, previous bladder surgery ● Uncooperative patients ● Mental impairment ● Tumor recurrence |
UDI IIQ (Thai Version) |
5.8 y ± 2.8 (Mean ± SD) |
Abbreviations: CRT, chemoradiotherapy; EBRT, external beam radiation therapy; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; ECOG, Eastern Cooperative Oncology Group; ESAS-r, Edmonton Symptom Assessment System; FIGO, International Federation of Gynecology and Obstetrics; FSFI, Female Sexual Function Index; IGBT, image-guided brachytherapy; IIQ, Incontinence Impact Questionnaire; MRI, magnetic resonance imaging; UDI, urogenital distress inventory; NACT, neoadjuvant chemotherapy; QoL, quality of life; RH, radical hysterectomy; RT, radiotherapy
Risk of Bias Assessment
Supplementary Table S1 (available in the online version only) shows the risk of bias assessments for the cohort and cross-sectional studies, while Supplementary Table S2 (available in the online version only) shows the risk of bias assessment for the phase II randomized clinical trial.
All but one cohort study were found to have a high risk of bias, which was attributed to significant confounding factors (tumor recurrence and comorbidities) that were not adjusted for in the analysis. Kirchheiner et al19 had low risk of bias since chronic disease was documented and patients with recurrence were censored from analysis. From the three cross-sectional studies, two were judged to be of low risk since key potential confounding factors were considered in the exclusion criteria, while one was of unclear risk since there was no mention of statistical adjustment for identified confounding factors. Both types of studies had similar issues, such as blinding or treatment specific, although this was justified and judged to not significantly impact the results of this systematic review. The included randomized clinical trial was found to be of low risk, since patients who developed recurrence during the study period were discontinued from the study.
Chemoradiotherapy Modalities
Nearly all the studies employed weekly cisplatin as the concurrent chemotherapy regimen (Table 2). A Thai study20 reported using tri-weekly cisplatin, or weekly or tri-weekly carboplatin, as alternative regimens. The EBRT technique was not specified in the studies, except for two,19,21 both of which used advanced, conformal EBRT techniques (3D CRT, IMRT). One study22 used standard 2D BRT, while three19,21,23 used IGBT. Furthermore, two reported using advanced hybrid ICISBT implants.19,21
|
Study |
Age (Median) |
2009 FIGO Stage |
Treatment |
Overall QoL |
QoL domains |
||
|---|---|---|---|---|---|---|---|
|
EBRT (Modality, %) |
Brachytherapy (Modality, %) |
Chemotherapy |
|||||
|
Phase 2 randomized clinical trial |
|||||||
|
Nunes de Arruda et al, 2020 |
45 |
IIB, 42% IIIA, 2% IIIB, 42% IVA, 13% |
● NS |
● 2D, majority |
Cisplatin (weekly, 40 mg/m2) |
Worse at 12 mos |
● IImproved in most aspects ● Worse peripheral neuropathy and menopausal symptoms. |
|
Cohort |
|||||||
|
Conway et al, 2020 |
46 |
IB, 33% II, 52% III, 15% |
● 3DCRT, 100% |
● IGBT, 100% (Intracavitary, 55%; ICISBT, 45%) |
Cisplatin (weekly, 40 mg/m2) |
Improved at 12 and 24 mo |
● Improved pain, drowsiness, depression and anxiety, appetite, and well-being. ● Worse tiredness and sense of well-being. |
|
Gargiulo et al, 2016 |
55.5 ± 11 (Mean ± SD) |
IB2, 4% II, 57% III, 35% IVA, 4% |
● NS |
● NS |
Cisplatin (weekly, 40 mg/m2) |
NA |
● Unimproved diarrhea and constipation at 2.5 y. |
|
Kirchheiner et al, 2016 |
49 |
IB, 17% II, 62% III, 20% IVA, 3% |
● IMRT, 26% ● 3DCRT, 74% |
● IGBT, 100% (Intracavitary, 61%; ICISBT, 39%) |
Cisplatin (weekly, 40 mg/m2) |
Improved at 12, 24, and 36 mo |
● Improved pain, constipation, and appetite. ● Decline in role and social functioning at 36 mo. |
|
Rai et al, 2014 |
48.2 (Mean) |
IB2, 6%, IIA, 3%, IIB, 86%, IIIB, 6% |
● NS |
● IGBT, 100% |
Cisplatin (weekly, 40 mg/m2) |
NAa |
● Resumed sexual activity in <50% at 12 mo. ● Unimproved dyspareunia, vaginal tightness, and shortening. |
|
Ljuca and Marošević, 2011 |
54 |
IIB: 100% |
● NS |
● NS (Intracavitary, 100%) |
Cisplatin (weekly, 40 mg/m2) |
NAa |
● Improved vaginal function at 12 mo. ● Unimproved overall sexual function at 12 mo. |
|
Cross-sectional |
|||||||
|
Daga et al, 2017 |
46.5 |
IIB, 13%, IIIA, 31%, IIIB, 33%, IVA, 23% |
● NS |
● NS (Intracavitary, 100%) |
NS |
NAa |
● Prevalent sexual function impairment. |
|
Prasongvej et al, 2017 |
50.4 ± 10.8 (Mean ± SD) |
I, 21% II, 47% III, 26% IV, 7% |
● NS |
●NS |
NS |
Prevalent global health impairment |
● Prevalent pain ● Prevalent physical and role function impairment. |
|
Katepratoom et al, 2014 |
55.1 ± 9.4 (Mean ± SD |
IB2, 6% IIA, 3% IIB, 46% IIB, 46% |
● NS |
● NS |
Cisplatin (weekly, 40 mg/m2 or tri-weekly, 100 mg/m2) Carboplatin (AUC 2 weekly, or AUC 6 tri-weekly) |
NAb |
● Prevalent lower urinary tract dysfunction (60%). |
Abbreviations: 2D, two-dimensional conventional; 3DCRT, three-dimensional conformal; CRT, chemoradiotherapy; EBRT, external beam radiation therapy; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; ESAS-r, Edmonton Symptom Assessment System; FIGO, International Federation of Gynecology and Obstetrics; FSFI, female sexual function index; IGBT, image-guided brachytherapy; ICISBT, intracavitary-interstitial brachytherapy; IIQ, Incontinence Impact Questionnaire; IMRT, intensity-modulated radiotherapy; NA, not applicable; NS, not stated; QoL, quality of life; RH, radical hysterectomy; AUC, area under the curve.
Quality of Life and Domains
The most commonly used tool was the European Organization for Research and Treatment for Cancer (EORTC) QLQ-C30,24 a validated tool for general health-related QoL among cancer patients consisting of symptom and functionality scales, that is used in combination with the QLQ-CX24, a validated tool for cervical cancer-specific QoL. These tools were used in five longitudinal and one cross-sectional studies. While two longitudinal studies19,21 reported improvement in overall QoL on follow-up, four reported worsening of symptoms: peripheral neuropathy,22 diarrhea,25 bladder dysfunction,25 menopausal symptoms,22 sexual activity,25 dyspareunia,23 and vaginal tightness and shortening.23 Only one study reported improvement in pain, appetite loss, and constipation.19 Most functions decline on long-term follow-up: role,19 social,19 and sexual.26 There was prevalent sexual inactivity23 and persistently impaired sexual function, despite improvement in vaginal function.26 A cross-sectional study27 reported prevalence of pain and impairment of global health, physical function, and role function among survivors at a mean follow-up of 4 years.
Gargiulo et al25 used the Incontinence Impact Questionnaire (IIQ-7),28,29 a validated 7-item questionnaire that assesses the different domains of QoL affected by urinary incontinence. They reported that incontinence was present in 36% of the cases and appeared to be mild and rarely disabling. Furthermore, it did not influence daily activities, entertainment, or the emotional state in nearly 50%.
Katepratoom et al20 similarly used the Thai version of the IIQ, as well as the Urogenital Distress Inventory (UDI).30 The UDI is a validated tool that assesses lower urinary tract symptom severity by evaluating three domains: irritative, obstructive/discomfort, and stress symptoms. They reported lower urinary tract dysfunction in 60% of the cases, with overall prevalence of lower urinary tract symptoms being documented in 77.1%. Stress incontinence was the most common type reported.
Daga et al31 used the Female Sexual Function Index (FSFI),32 a validated tool that measures six domains of sexual function and identifies patients with female sexual arousal disorder (given a cutoff score of <26.55). They reported decreased sexual function among survivors, reflected by an overall average score of 11.84, which is indicative of female sexual arousal disorder.
Conway et al21 used the Revised Edmonton Symptom Assessment System (ESAS-r),33 a validated self-report tool for symptom intensity in advanced cancer. They reported that, while symptoms improved over time, tiredness and lack of well-being remained to be most distressing on follow-up.
Clinical Determinants of Overall QoL and QoL Domains
Three studies reported QoL assessments that were specific to age (Table 2).21,22,26 Conway et al21 noted that more problems were encountered by older patients compared with younger patients, signifying that age may be an important determinant of both distress and overall QoL. Furthermore, Nunes de Arruda et al22 noted that age affected scores on body image, sexual activity, and menopausal symptoms.
Conway et al21 reported on the correlation of QoL with the FIGO stage (Table 2). Stage predicted symptom severity, distress, QoL impairment, functional impairment, and complications.
Late Toxicities
While rare (2%), grade 4 gastrointestinal and genitourinary toxicities, such as small bowel obstruction and a vesico-vaginal fistula, were documented.21 Peripheral neuropathy appeared immediately after treatment and worsened over time,22 while lymphedema and dyspnea developed more gradually after treatment.19 Uncommonly (7%), grade 3 to 4 musculoskeletal toxicities, in the form of insufficiency fractures, were also recorded.21
Discussion
Advancements in the management of LACC in the past decade have resulted in improved disease control and survival. This increased survival means that post-treatment morbidity and QoL have become more relevant. Indeed, QoL has emerged as an important clinical end point in trials.22,34,35
The EORTC QLQ-C30 and QLQ-CX24 are well-validated36 and have been widely used in clinical trials. Their availability in several languages allows use in different countries. As we begin to understand better long-term QoL among cervical cancer survivors, the EORTC has begun developing and validating QoL tools specific to cancer survivors. Currently, survivorship modules for breast, colorectal, and prostate cancer are under development.37
In our review, the EORTC QLQ-C30 and CX24 were the most commonly used tools in evaluating health-related and disease-specific QoL.19,22,23,25,26,27 Two papers characterized the temporal evolution of health-related QoL among LACC survivors.19,21 One reported improvement at 12, 24, and 36 months.19 In this cohort, 3DCRT or IMRT, and IGBT were used entirely, and hybrid ICISBT was an available modality. The other employed 2D BRT in the majority of its cohort and reported worsened QoL at 12 months.22 A cross-sectional study reports that, at 4 years, global health impairment among survivors remains prevalent.27
Significant QoL impairment was documented in four domains: gastrointestinal, genitourinary, sexual, and psychosocial.
Gastrointestinal dysfunction is among the most prevalent and persistent.19,25 While constipation tended to improve, diarrhea persisted beyond 24 months. Diarrhea, due to chronic radiation enteropathy,38 needs to be differentiated from pseudodiarrhea, or increased stool frequency that could be due to chronic radiation proctopathy.39 Radiation enteropathy is associated with chronic inflammation of the bowels, leading to malabsorption and malnutrition, not unlike bowel inflammatory disease. Radiation proctopathy is associated with mucosal atrophy and telangiectasia and decreased capacity of the rectal vault due to fibrosis. This could manifest as increased stool frequency and rectal bleeding. Preventative interventions include agents that could counteract the oxidative injury of radiation to these organs, such as vitamin E and analogs, superoxide dismutase and mimetics, and amifostine; and agents that modulate pathophysiological responses to radiation injury, such as immune-modulating drugs (misoprostol), enterotrophic agents, and compounds that modulate intraluminal contents (sucralfate, somatostatin). These interventions are either investigational, of poor practicality, or of uncertain safety or effectiveness.38 Thus, preventative strategies remain largely limited to decreasing radiation dose to small bowels and the rectum, which entails improvement of radiation delivery.40,41 Compensatory and supportive strategies include dietary modification and nutritional supplementation.42
Two studies primarily investigated genitourinary function. Gargiulo et al reported urinary incontinence to be common but rarely disabling at 36 months.25 Conversely, Katepratoom et al,20 using the UDI, found lower urinary tract symptoms to remain prevalent (77%), stress incontinence being the most common, among survivors at 6 years. Importantly, lower urinary tract dysfunction was present in 60%. Fokdal et al reported rates of mild to moderate and severe to life-threatening urinary morbidity to be 57.5 and 5.1% respectively, in a cohort treated entirely with 3DCRT or IMRT, and IGBT, at 27 months. Documented end points were similar, with frequency, incontinence, and cystitis occurring most frequently. Radiotherapy dose-volume constraints were proposed for the prevention of urinary morbidity.43 Parasympathetic nerve damage and sympathetic denervation, as well as radiation injury leading to fibrosis and sclerosis, have been associated with these complications following typical treatment regimens.44 Other proposed preventative strategies include identifying co-existing anatomical abnormalities prior to treatment and implementing preventive pelvic floor muscle exercises to maintain muscle strength.45,46 Pharmacologic interventions, such as non-steroidal anti-inflammatory drugs (ibuprofen, solifenacin) for dysuria, antispasmodics (oxybutynin, tolterodine) for detrusor instability, or local vaginal estrogen can also be prescribed.42,47 Early identification and management of related complications, (UTI, ureteral strictures, and fistulae), and urogynecologic referral are recommended compensatory and supportive strategies.47
Three studies investigated primarily sexual function. Rai et al reported sexual inactivity to remain prevalent, and dyspareunia and vaginal tightness and shortening to persist among LACC survivors at 12 months.23 Even in a cohort of only Stage IIB cervical cancer survivors, Ljuca and Marošević reported that overall sexual function remained unimproved at 12 months, despite improved vaginal function.26 Furthermore, Daga et al reported that sexual function impairment was prevalent on follow-up beyond 2 years.31 Kirchheiner et al have proposed radiation dose-volume parameters to prevent vaginal toxicity48 and, in an abstract-only article, they reported sexual QoL outcomes in their updated cohort.49 At 36 months, sexual activity was reported in more than half. Among these, compromised sexual enjoyment was reported in nearly half, and sexual function problems in around one-third. Supportive strategies for vaginal dryness or stenosis include lubricants and estrogen creams, and vaginal dilators.31 In addition to vaginal toxicity, pelvic radiotherapy results in ovarian dysfunction and onset of menopausal symptoms, such as hot flashes and fatigue,50 which could in turn contribute to diminished sexual desire, arousal, and satisfaction. Management includes hormone replacement therapy to decrease symptoms and improve QoL.22 Among survivors with sexual function issues, the presence of symptoms of distress, anxiety, depression or other psychological concerns needs to be evaluated.42
One study investigated primarily symptoms of distress. Conway et al reported that, while most psychological symptoms (pain, drowsiness, depression, anxiety) improved at 12 and 24 months, tiredness and a lack of sense of well-being tended to worsen.21 Furthermore, Kirchheiner et al reported a decline in role and social function at 36 months,19 and Prasongvej et al reported prevalent physical and role function impairment at 4 years.27 Patient education could alleviate distress, anxiety, and depression, improve their sense of empowerment, and foster a more active and collaborative role in their own management, which has been shown to improve QoL. Social reintegration and opportunities for employment and engagement could foster a sense of well-being, purpose, and belongingness. The National Cancer Comprehensive Network has published patient education resources for cancer survivors.51
Several late toxicities are worth further discussing: peripheral neuropathy, lymphedema, and insufficiency fractures.
Peripheral neuropathy was reported to develop early and persist at 12 months,22 highlighting the long-term effects of platinum therapy. Further, the recognition of cisplatin-related ototoxicity has been well-documented for head-and-neck cancers, but less so for cervical cancers.52
Lymphedema was noted to develop more gradually.19 Lower-limb and perineal lymphedema is a documented complication of both surgery (surgical nodal staging and therapeutic lymphadenectomy) and radiotherapy for cervical cancer.53 Ferrandina et al54 noted early worsening of lymphedema, both in early cervical cancer patients who underwent surgery and in LACC patients treated with CRT followed by surgery. However, lymphedema among the latter tended to peak higher (28.3% relative to baseline) compared with the former (14.6%), highlighting the combined effect of the two modalities. The increasing use of positron emission tomography scan in staging has led to diminished use of surgical nodal staging. On the other hand, the increasing use of nodal boost, as well as ICISBT, may lead to higher lymphedema rates. In a cohort treated entirely by 3DCRT or IMRT and IGBT, and that utilized nodal boosts as indicated, Najjari et al55 reported an incidence rate of 22.2% at 27 months, and obesity and invasive nodal staging to be significant determinants. On the other hand, an extended EBRT field was associated with a non-significant correlation, and nodal boost, no correlation, with lymphedema. The burden of lymphedema could be alleviated by early identification, self-care management, supportive interventions (compression garments, manual drainage), and rehabilitative interventions (range-of-motion exercises).42
Musculoskeletal events, specifically insufficiency fractures, although uncommon, have been reported on longer-term follow-up,21 supporting the practice of limiting radiation doses to the femurs, as well as the importance of vitamin D supplementation, osteoporosis screening, and hormonal replacement therapy.42
Limitations
The number and quality of studies included in our review reflect the relative paucity of high-quality literature on long-term QoL among LACC survivors. Of the nine papers included in this review, one19 is a multinational study; six20,21,22,25,26,27 come from upper-middle or high income countries, namely, Brazil, Canada, Italy, Bosnia and Herzegovina, and Thailand; and two23,31 come from a lower-middle income country, India.56 The studies include three small (<50) cross-sectional studies,20,27,31 one (n = 67) retrospective study,21 and three small (<60) prospective studies.23,25,26 Only the multinational study19 reported multiple QoL assessments beyond the first year after completion of therapy, while only two cross-sectional studies from Thailand20,27 provide QoL data beyond 3 years of survivorship.
Given these, information on determinants for QoL or its domains that could be derived from this review are limited. Age was a determinant for body image, sexual activity, and menopausal symptoms.22 Age, stage, and baseline symptom distress levels were determinants for long-term symptom distress.21 Baseline physical condition, which was measured using the EORTC QLQ-C30 functioning scale, predicted the development of dyspnea.19 Age and baseline physical condition impact QoL domains among survivors differently. Aside from age-related differences in physical, social, and sexual functional demands, CRT exposure at a younger age could lead to premature menopause. This could mean increased burden of menopausal symptoms and risks for cardiovascular and skeletal events. Among the elderly, the late effects of CRT compound the already complex interactions of co-existing comorbidities.
Recommendation
The multinational, mostly European, group has published updated, outcome-specific analyses directed toward identifying optimal radiotherapy dose-volume parameters, based on their growing prospective observational cohort.15,40,50,55 The group has also published a review article on systematic assessment and registration of morbidity outcomes.57 This demonstrates the feasibility of establishing collaborative regional prospective registries to generate meaningful data that are appropriate to each region or locality and could inform clinical decision-making, both in the active treatment and post-treatment surveillance, toward improving QoL outcomes among LACC survivors.
Conclusion
Advances in the delivery of definitive CRT for LACC have resulted in improved disease control and survival, and QoL and survivorship have become paramount. Published quality data on QoL are limited. Cohorts that employ advanced EBRT and BRT techniques find overall QoL to improve in the first 3 years from treatment completion. Nevertheless, gastrointestinal, genitourinary, sexual, and psychosocial function impairment persist on the long-term. Other late toxicities worth noting include peripheral neuropathy, lower limb edema, and insufficiency fractures. Collaborative prospective registries could generate data that could clarify determinants of QoL and inform treatment and surveillance strategies toward improving QoL among LACC survivors.
Conflict of Interest
None declared.
Supplementary Material
Funding None.
References
- Cervical cancer [Internet] World Health Organization. World Health Organization. Available at: https://www.who.int/health-topics/cervical-cancer#tab=tab_1
- [Google Scholar]
- Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(02):e191-e203.
- [Google Scholar]
- Human Papillomavirus and Related Diseases Report: WORLD. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Available at: https://www.hpvcentre.net/statistics/reports/XWX.pdf/
- [Google Scholar]
- Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161.
- [Google Scholar]
- Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153.
- [Google Scholar]
- Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137-1143.
- [Google Scholar]
- Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(05):1339-1348.
- [Google Scholar]
- Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000 Apr;18(8):1606-13. doi: 10.1200/JCO.2000.18.8.1606. PMID: 10764420
- [Google Scholar]
- Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802-5812.
- [Google Scholar]
- Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol. 2018;36(24):2538-2544.
- [Google Scholar]
- Management of nodal disease in advanced cervical cancer. Semin Radiat Oncol. 2019;29(02):158-165.
- [Google Scholar]
- Dose-volume effects in pathologic lymph nodes in locally advanced cervical cancer. Gynecol Oncol. 2018;148(03):461-467.
- [Google Scholar]
- Phase III RCT of postoperative adjuvant conventional radiation (3DCRT) versus IGIMRT for reducing late bowel toxicity in cervical cancer (PARCER) (NCT01279135/CTRI2012/120349): results of interim analyses. Int J Radiat Oncol Biol Phys 2015;93(03):
- [Google Scholar]
- Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120(03):428-433.
- [Google Scholar]
- Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120(03):434-440.
- [Google Scholar]
- The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Support Care Cancer. 2014;22(04):1089-1096.
- [Google Scholar]
- Symptom research in gynecologic oncology: a review of available measurement tools. Gynecol Oncol. 2010;119(02):384-389.
- [Google Scholar]
- Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat Rev. 2003;29(06):471-488.
- [Google Scholar]
- Health-related quality of life in locally advanced cervical cancer patients after definitive chemoradiation therapy including image guided adaptive brachytherapy: an analysis from the EMBRACE Study. Int J Radiat Oncol Biol Phys. 2016;94(05):1088-1098.
- [Google Scholar]
- Lower urinary tract dysfunction and quality of life in cervical cancer survivors after concurrent chemoradiation versus radical hysterectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2014;25(01):91-96.
- [Google Scholar]
- Long-term patient-reported distress in locally advanced cervical cancer patients treated with definitive chemoradiation. Clin Transl Radiat Oncol. 2020;23:1-8.
- [Google Scholar]
- Quality of life of locally advanced cervical cancer patients after neoadjuvant chemotherapy followed by chemoradiation versus chemoradiation alone (CIRCE trial): a randomized phase II trial. Int J Gynecol Cancer. 2020;30(06):749-756.
- [Google Scholar]
- Vaginal dose, toxicity and sexual outcomes in patients of cervical cancer undergoing image based brachytherapy. Asian Pac J Cancer Prev. 2014;15(08):3619-3623.
- [Google Scholar]
- The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(05):365-376.
- [Google Scholar]
- Long-term toxicity and quality of life in patients treated for locally advanced cervical cancer. Oncology. 2016;90(01):29-35.
- [Google Scholar]
- Impact of chemoradiotherapy on vaginal and sexual function of patients with FIGO IIb cervical cancer. Bosn J Basic Med Sci. 2011;11(01):62-64.
- [Google Scholar]
- Quality of life in cervical cancer survivors and healthy women: Thai Urban Population Study. Asian Pac J Cancer Prev. 2017;18(02):385-389.
- [Google Scholar]
- Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995;14(02):131-139.
- [Google Scholar]
- Test-retest reliability, validity, and sensitivity to change of the urogenital distress inventory and the incontinence impact questionnaire. Neurourol Urodyn. 2002;21(06):534-539.
- [Google Scholar]
- Validation of Thai version of the urogenital distress inventory and incontinence impact questionnaires. Chulalongkorn Medical Journal. 2012;56(01):37-50.
- [Google Scholar]
- Sexual function in cervical cancer survivors after concurrent chemoradiotherapy. Middle East J Cancer. 2017;8(03):151-154.
- [Google Scholar]
- The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(02):191-208.
- [Google Scholar]
- Modified Edmonton Symptom Assessment System including constipation and sleep: validation in outpatients with cancer. J Pain Symptom Manage. 2015;49(05):945-952.
- [Google Scholar]
- CALLA: efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer. 2020;30(07):1065-1070.
- [Google Scholar]
- Bethesda (MD): National Library of Medicine (US) Identifier: NCT01414608, Cisplatin and Radiation Therapy with or without Carboplatin and Paclitaxel in Patients with Locally Advanced Cervical Cancer; 2011 Aug 11. Available at: https://clinicaltrials.gov/ct2/show/NCT01414608. Available at: March 24, 2021
- [Google Scholar]
- The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer. 2006;107(08):1812-1822.
- [Google Scholar]
- Survivorship: EORTC—Quality of Life. EORTC. Accessed March 24, 2021 at: https://qol.eortc.org/questionnaire/surv111/
- [Google Scholar]
- Radiation enteropathy—pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11(08):470-479.
- [Google Scholar]
- Dose-volume effects and risk factors for late diarrhea in cervix cancer patients after radiochemotherapy with image guided adaptive brachytherapy in the EMBRACE I study. Int J Radiat Oncol Biol Phys. 2021;109(03):688-700.
- [Google Scholar]
- Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: physician- and patient reported outcome from the EMBRACE study. Radiother Oncol. 2018;127(03):431-439.
- [Google Scholar]
- Survivorship (version 1.2021) NCCN; 2021. Accessed March 23, 2021 at: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf
- [Google Scholar]
- Physician assessed and patient reported urinary morbidity after radio-chemotherapy and image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother Oncol. 2018;127(03):423-430.
- [Google Scholar]
- Urological complications after treatment of cervical cancer. Nat Rev Urol. 2014;11(02):110-117.
- [Google Scholar]
- Prevalence and management of (non-fistulous) urinary incontinence in women following radical hysterectomy for early stage cervical cancer. Eur J Gynaecol Oncol. 2001;22(01):26-30.
- [Google Scholar]
- Pre-rehabilitation of the pelvic floor before radiation therapy for cervical cancer: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2020;31(11):2411-2418.
- [Google Scholar]
- Cervical cancer: an overview of pathophysiology and management. Semin Oncol Nurs. 2019;35(02):166-174.
- [Google Scholar]
- Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol. 2016;118(01):160-166.
- [Google Scholar]
- Sexual activity and vaginal functioning in patients with locally advanced cervical cancer following Definitive Radiochemotherapy and Image-Guided Adaptive Brachytherapy (EMBRACE Study) Int J Radiat Oncol Biol Phys. 2019;105(01):S51-S52.
- [Google Scholar]
- Fatigue, insomnia and hot flashes after definitive radiochemotherapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: An analysis from the EMBRACE study. Radiother Oncol. 2018;127(03):440-448.
- [Google Scholar]
- Survivorship care for cancer-related late and long-term effects. NCCN; 2020. Available at: https://www.nccn.org/patients/guidelines/content/PDF/survivorship-crl-patient.pdf. March 23, 2021
- [Google Scholar]
- Age-corrected hearing loss after chemoradiation in cervical cancer patients. Strahlenther Onkol. 2018;194(11):1039-1048.
- [Google Scholar]
- Lower body lymphedema in patients with gynecologic cancer. Anticancer Res. 2017;37(08):4005-4015.
- [Google Scholar]
- Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: a prospective, longitudinal study. Gynecol Oncol. 2012;124(03):389-394.
- [Google Scholar]
- Physician assessed and patient reported lower limb edema after definitive radio(chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: a report from the EMBRACE study. Radiother Oncol. 2018;127(03):449-455.
- [Google Scholar]
- World Bank Country and Lending Groups. The World Bank. Accessed March 24, 2021 at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bankcountry-and-lending-groups
- [Google Scholar]
- Initiatives for education, training, and dissemination of morbidity assessment and reporting in a multiinstitutional international context: Insights from the EMBRACE studies on cervical cancer. Brachytherapy. 2020;19(06):837-849.
- [Google Scholar]







