Translate this page into:
A bibliometric analysis of CiteSpace software-based immunotherapy for the treatment of diffuse large B-cell lymphoma
Corresponding author: Dr. Weichen Si, Department of Acupuncture and Moxibustion, Beijing Direction Community Health Service Station, Beijing, China. weichensi14@outlook.com
-
Received: ,
Accepted: ,
How to cite this article: Si W. A bibliometric analysis of CiteSpace software-based immunotherapy for the treatment of diffuse large B-cell lymphoma. Asian J Oncol, 2023;9:1.
Abstract
Objectives
To analyze the literature data and research status of immunotherapy for the treatment of diffuse large B-cell lymphoma since the establishment of the Web of Science (WOS) core database.
Material and Methods
The WOS core database was searched for literature related to immunotherapy for diffuse large B-cell lymphoma, and the included literature was formatted, cleaned, node merged, and analyzed using CiteSpace software. Based on the parameters set, the included literature was analyzed for trends in publications, author publications and inter-author collaborations, national publications, global institutional publications and inter-institutional collaborations, citations, keyword co-occurrence, keyword emergence, and keyword clustering. The final visual knowledge map was created.
Results
A total of 370 articles were selected for inclusion. The highest number of annual publications was in 2021. Four individuals, Marconato Laura; Ansell, Stephen M; Xiao Min; and Aresu Luca, were the most published scholars. The United States with 152 publications was the country with the highest number of publications. Benjamin J is the most cited scholar in this field. The top three most cited keywords were “expression,” “diffuse large b-cell lymphoma,” and “rituximab.” “Bone marrow transplantation” was the first and longest-running keyword. “Cancer immunotherapy,” “resistance,” and “cytokine release syndrome” are still hot topics. The keyword clusters “pd-l1,” “antibody-based,” “immunotherapy,” and “cd19” were the main clusters studied.
Conclusion
After visualization and analysis, the recent research and hot trends in the field of immunotherapy for diffuse large B-cell lymphoma were reviewed using knowledge mapping and further presented in a visualized form, providing a reference for further development of related research in the future.
Keywords
Immunotherapy
Diffuse large B-cell lymphoma
CiteSpace
Metrology
INTRODUCTION
The application of immunotherapy in the treatment of diffuse large B-cell lymphoma is one of the hot topics in the field of tumor therapy, and it is of great significance to the bibliometrics research in this field. Specifically, this study can help readers understand the research progress and trend of immunotherapy in the treatment of diffuse large B-cell lymphoma, and provide scientific basis and reference for clinical practice. Analyzing and evaluating the quality and quantity of existing research helps us understand the overall situation of research in this field, helps to find the defects and deficiencies in research, and provides the direction of improvement and perfection for future research. It will provide important reference information for policy makers, managers, researchers, and investors in relevant research fields to help them formulate more scientific and rational development strategies and plans and promote further development and research in this field. To provide the academia and the public with the latest information on research progress in this field, the public’s attention and awareness of this field should be improved, and the achievements and technologies in this field should be popularized.
MATERIAL AND METHODS
Etiology of diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is a type of non-Hodgkin’s lymphoma (NHL) that originates from B cells in the lymphatic system.[1] The causes of DLBCL are not fully understood, but several factors have been identified that may increase the risk of developing this condition: Genetic mutations: Certain genetic mutations have been associated with an increased risk of developing DLBCL, including mutations in genes that regulate cell growth and division.[2] Immune system dysfunction: DLBCL may occur when the immune system is weakened, such as in people with HIV/AIDS or those who have undergone an organ transplant and are taking immunosuppressive drugs.[3] Viral infections: Some viral infections, such as Epstein–Barr virus (EBV) and human herpesvirus 8 (HHV-8), have been linked to an increased risk of developing DLBCL. Environmental factors: Exposure to certain environmental toxins, such as pesticides, may increase the risk of developing DLBCL.[4] Age: DLBCL is more common in older adults, with the average age at diagnosis being around 65 years. It is important to note that while these factors may increase the risk of developing DLBCL, not everyone who is exposed to these risk factors will develop the disease.[5] In addition, many people with DLBCL have no identifiable risk factors, and the exact cause of their lymphoma is unknown.
Pathogenesis of diffuse large B-cell lymphoma
DLBCL involves the transformation of B cells in the lymphatic system into malignant cells that grow and divide uncontrollably.[6] This can result in the formation of tumors and the spread of cancer to other parts of the body.[6] DLBCL arises from a particular type of B cell known as the germinal center B cell, which normally plays a role in the immune response to infection. However, in DLBCL, these cells acquire mutations that disrupt their normal function, causing them to proliferate abnormally.[7] There are several genetic alterations that have been associated with DLBCL pathogenesis.[5] The most common genetic alteration is a translocation between the BCL2 and IGH genes, which results in overexpression of the BCL2 protein 2 . This protein helps to prevent apoptosis (programmed cell death) and can promote the survival of cancer cells.[8] Other genetic alterations that can contribute to DLBCL pathogenesis include mutations in genes involved in B cell signaling pathways, as well as alterations in genes that regulate cell growth and division.[4] In addition to genetic alterations, DLBCL pathogenesis can also be influenced by epigenetic changes, such as alterations in DNA methylation or histone modifications, which can affect gene expression[1]. The precise sequence of events that lead to DLBCL pathogenesis can be complex and may involve interactions between multiple genetic and environmental factors.[9] However, a better understanding of the underlying molecular mechanisms involved in DLBCL pathogenesis may lead to the development of more effective treatments for this disease.[2,3]
The treatment of diffuse large B-cell lymphoma
DLBCL typically depends on several factors, including the patient’s age and overall health, the stage and aggressiveness of the lymphoma, and the presence of any genetic abnormalities.[10] First-line treatment for DLBCL typically involves combination chemotherapy, which is often administered with the monoclonal antibody rituximab.[10,11] The most commonly used chemotherapy regimen for DLBCL is called R-CHOP, which includes rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.[12] This regimen is typically administered every 21 days for six cycles. For patients who are not candidates for R-CHOP, alternative chemotherapy regimens, such as EPOCH or dose-adjusted R-EPOCH, may be used. In some cases, radiation therapy may be added to the treatment regimen. Second-line treatment options for DLBCL include high-dose chemotherapy followed by autologous stem cell transplantation (ASCT), which involves the use of the patient’s own stem cells to replace the damaged bone marrow.[13] Other options may include targeted therapies, such as the monoclonal antibody drug brentuximab vedotin or the immunomodulatory drug lenalidomide. Adjuvant therapy, which is given after the initial treatment, may include radiation therapy or maintenance therapy with rituximab.[14] These therapies are designed to help prevent the recurrence of the lymphoma. For patients with relapsed or refractory DLBCL, salvage chemotherapy followed by ASCT may be considered.[15] Other treatment options for relapsed or refractory DLBCL may include targeted therapies, such as the BTK inhibitor ibrutinib or the PI3K inhibitor idelalisib, as well as immunotherapy, such as CAR T-cell therapy.[16]
Overview of immunotherapy
Immunotherapy is a type of cancer treatment that harnesses the body’s own immune system to fight cancer.[17] It is designed to stimulate the immune system to recognize and attack cancer cells, thereby slowing or stopping the growth and spread of cancer. One of the major advantages of immunotherapy is its specificity.[18] Unlike traditional cancer treatments, such as chemotherapy, which can kill both cancerous and healthy cells, immunotherapy drugs are designed to target cancer cells specifically.[19] This means that they can be more effective in treating cancer while minimizing damage to healthy cells. Another advantage of immunotherapy is its potential to provide long-lasting responses.[20] In some cases, immunotherapy has been shown to cause complete remission, meaning that the cancer is no longer detectable in the body, and patients can remain cancer-free for years after treatment. Moreover, immunotherapy is a relatively new field, and researchers continue to develop and refine new immunotherapy treatments.[21] This means that there is potential for even greater advancements in the future, with the development of more effective and targeted immunotherapies.
Research Methodology
Bibliometric analysis involves the use of quantitative methods to analyze scientific publications in order to evaluate the impact of a particular field or subject area.[22] In the case of DLBCL, a type of blood cancer that affects B cells, bibliometric analysis could provide insights into the research trends, collaborations, and impact of scientific publications related to this disease.[23] One potential implication of bibliometric analysis of DLBCL is that it could help identify the most influential authors, institutions, and countries in the field. This could inform decisions about where to allocate funding and resources for research, as well as identify potential collaborations and partnerships.[24] Another implication is that bibliometric analysis could provide insights into the research trends and gaps in DLBCL.[25] For example, by analyzing the keywords used in DLBCL publications, it may be possible to identify emerging areas of research or areas where there is a lack of research. In addition, bibliometric analysis could help evaluate the impact of DLBCL research on clinical practice and patient outcomes.[22,23] By analyzing citations of DLBCL publications in clinical guidelines and other publications, it may be possible to assess the extent to which research has influenced clinical decision-making and patient care. Overall, bibliometric analysis of DLBCL could provide valuable insights into the research trends, collaborations, and impact of scientific publications related to this disease, which could inform future research and clinical practice.[25]
This research uses Web of Science (WOS) as the data source. Search subject terms: TS = (“Diffuse Large B-cell Lymphoma” OR “ALL”) AND TS = (“immunotherapy”). The search time was set from library creation to December 2022. The retrieved literature was manually screened to exclude literature with titles, abstracts, content not clearly relevant to DLBCL, conference papers, scientific and technical results, and newspapers. Finally, the relevant documents that met the criteria were exported in plain text format, named “download_**.txt” and imported into CiteSpace (6.1.R3) for format conversion, data cleaning, node merging, and data analysis. The time slice was 1 year, and the source of the subject terms was selected by default. According to each setting parameter, the included related literature was analyzed for publication trend, author publication and inter-author collaboration, publication volume by country, publication volume by global institutions and inter-institutional collaboration, literature cited, keyword co-occurrence, keyword emergence, keyword clustering, and the network knowledge graph of DLBCL was drawn. The software prompts are combined with manual literature reading and information integration for in-depth analysis of the mapping information.
CiteSpace is a bibliometric analysis software tool that enables the visualization and analysis of citation networks in scientific literature.[26] It is widely used in various fields, including science, technology, and medicine, to identify research trends, key contributors, and emerging areas of research. CiteSpace uses a variety of metrics and algorithms to identify important research themes and patterns of collaboration among researchers.[27] The software can be used to identify the most frequently cited papers in a particular field, the most influential authors, and the most productive institutions. In addition, it can analyze co-citation networks to identify clusters of related research topics, and it can identify bursty keywords that are becoming increasingly popular in a given field. The research methodology employed by CiteSpace is important because it provides a systematic, objective, and quantitative approach to bibliometric analysis. By analyzing citation networks, CiteSpace can reveal patterns and trends in scientific research that may not be readily apparent through other methods.[28] This information can be used to inform research policy, identify promising areas for future research, and evaluate the impact of scientific publications. The use of CiteSpace can also help researchers to identify potential collaborators and to stay up-to-date with the latest research in their field. By analyzing the citation patterns of researchers and institutions, CiteSpace can help to identify important research trends and collaborations that might not be immediately apparent.[29] Overall, the research methodology employed by CiteSpace is an important tool for bibliometric analysis and can provide valuable insights into the structure and evolution of scientific research.
RESULTS
Results of the trend analysis of publications
A total of 370 articles related to immunotherapy for DLBCL were obtained from the WOS database of 2022. The trend and distribution of the literature on DLBCL obtained from the CiteSpace analysis is shown in Figure 1, where the first appearance of DLBCL in the last 20 years was in 2003. The years 2003–2010 were the initial stage of DLBCL research, with less than 10 articles published per year and few relevant research results; 2011–2018 saw a slight increase in research literature (11–32 articles) and a significant upward trend; 2019–2022: After a small decrease, the annual number of articles peaks at 62 in 2021 and then decreases slightly in 2022. The total number of papers on immunotherapy for DLBCL from the web of science core build to 2022 is 370. New high-level research on immunotherapy for DLBCL is emerging, and there is still much room for future research development.
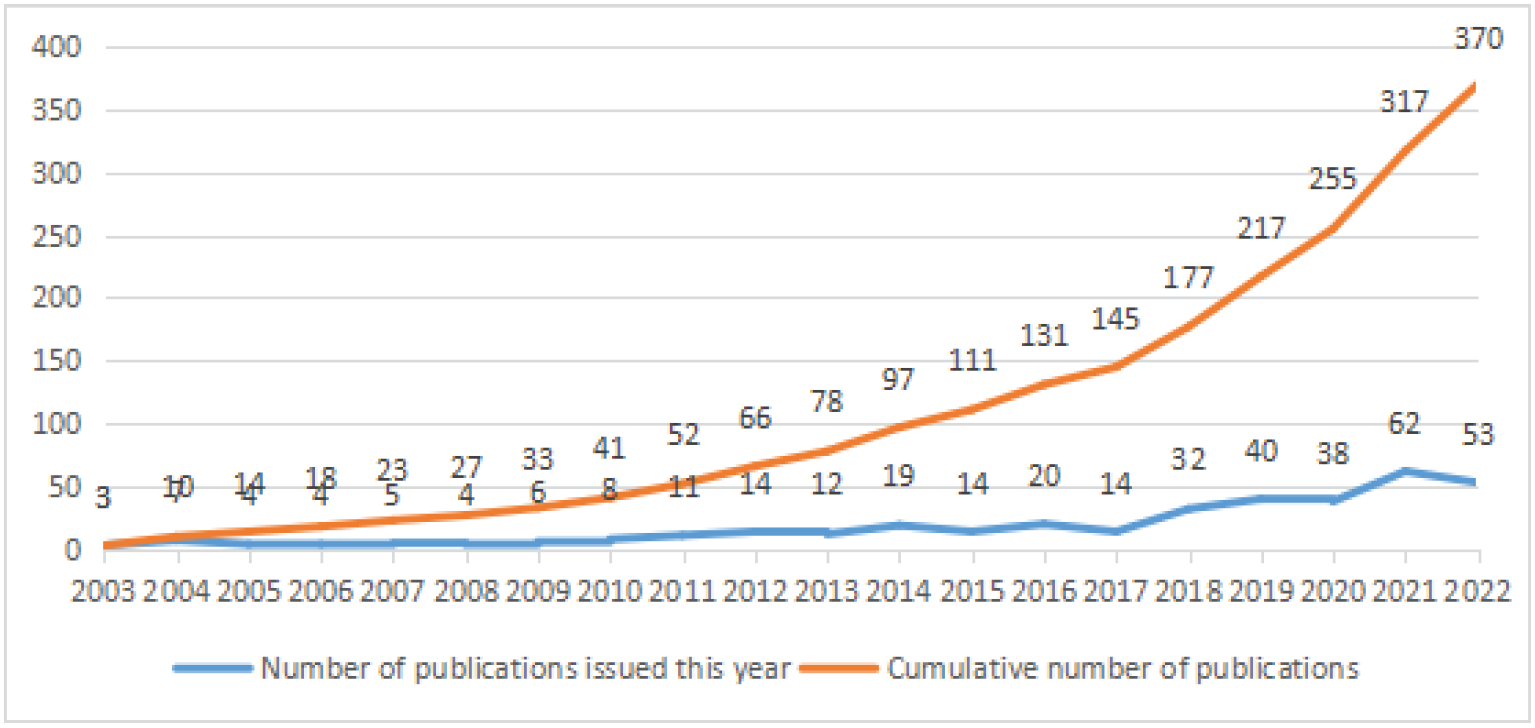
-
Research publications on immunotherapy for diffuse large B-cell lymphoma.
Results of the analysis of authorship and collaboration between authors
A total of 496 authors were included in the resulting map, with the highest number of publications being six. The four authors with the highest number of publications were Marconato Laura; Ansell, Stephen M; Xiao Min; and Aresu Luca. The number of articles published by Stefano was five. The number of articles published by Dybkaer Karen, Tzankov Alexandar, Xu-monette, and Zijun Y was four. The number of authors with three articles is 22, the number of authors with two articles is 56, and the number of authors with one article is 405. Marconato Laura; Ansell, Stephen M; Xiao Min; and Aresu Luca are the most influential authors in the field of immunotherapy for DLBCL. Each node in Figure 2 represents an author. The larger the node, the greater the number of publications, and the thicker the line, the closer the connection between the authors. The 10 scholars with the largest number of publications are shown in Table 1.
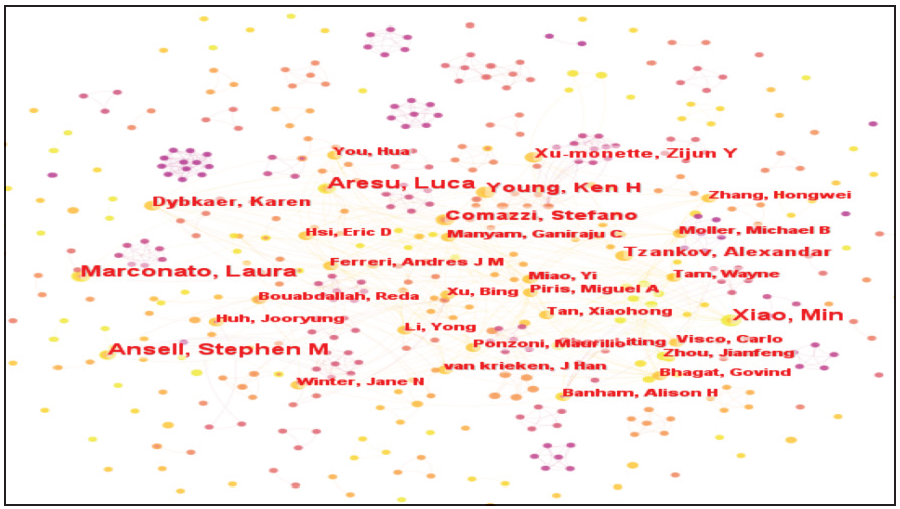
-
Co-presentation of core authors in the research literature on immunotherapy for diffuse large B-cell lymphoma.
| Author | Year | Number of publications |
|---|---|---|
| Marconato Laura | 2014 | 6 |
| Ansell Stephen M | 2009 | 6 |
| Xiao Min | 2019 | 6 |
| Aresu Luca | 2014 | 6 |
| Young Ken H | 2018 | 5 |
| Comazzi Stefano | 2014 | 5 |
| Dybkaer Karen | 2017 | 4 |
| Tzankov Alexandar | 2019 | 4 |
| Xu-monette Zijun Y | 2018 | 4 |
| Li Yong | 2019 | 3 |
Analysis of the volume of articles published by country
As shown in Table 2 and Figure 3, there is a wide disparity in the number of articles published in different countries, with the United States ranking first with the highest number of articles at 152, creating a fault line between the second place. The second highest number of articles was published by PEOPLES R CHINA with 71 articles, FRANCE with 40 articles, and GERMANY with 37 articles. In summary, there is a wide disparity in research on diffuse large B-cell lymphoma across countries, with the United States having the most research, more than twice as many as the second most published country, suggesting that the United States is significantly ahead of the world in research in this area. In developing countries other than China, there is less research on immunotherapy for DLBCL and further improvement is needed.
| Country | Year | Number of articles | Country | Year | Number of articles |
|---|---|---|---|---|---|
| USA | 2003 | 152 | England | 2004 | 26 |
| Peoples R China | 2011 | 71 | Spain | 2004 | 22 |
| France | 2006 | 40 | Switzerland | 2012 | 21 |
| Germany | 2004 | 37 | Canada | 2003 | 19 |
| Italy | 2006 | 35 | Netherlands | 2003 | 15 |
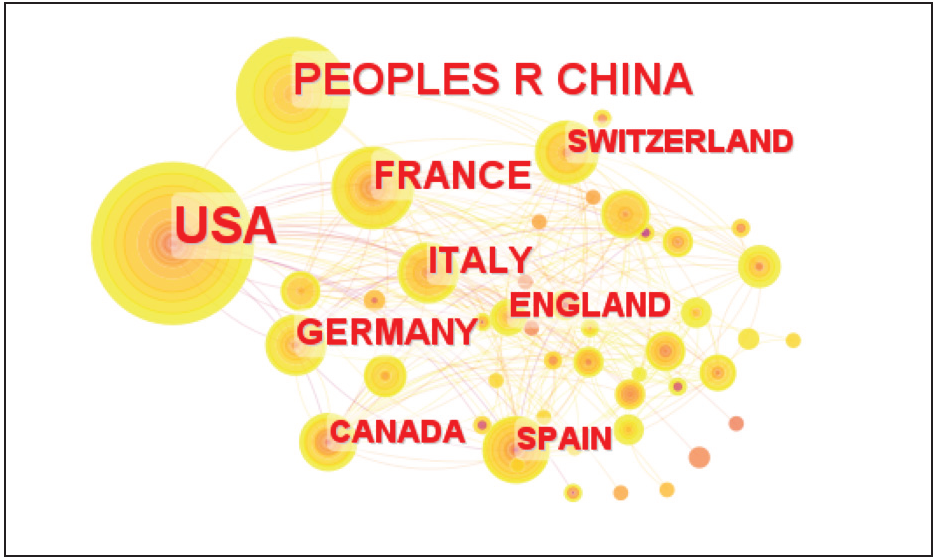
-
Volume of relevant literature by country.
Results of the analysis of the number of articles published by institutions and inter-institutional collaboration
A total of 328 institutions were included in the 370 included papers, and the top 10 institutions in terms of number of publications are shown in Table 3. A visual analysis of the collaboration network was conducted [Figure 4]. A total of 51 nodes and 276 links were obtained from the analysis, indicating that there are a large number of institutions studying immunotherapy for DLBCL and that the institutions are closely linked to each other. For DLBCL research, inter-institutional coordination and collaboration leads to more impactful research results. UTMD Anderson Cancer Center with 24 articles has published the most number of articles. Harvard University has published 20 articles, and Institut National de la Sante et de la Recherche Medicale (Inserm) has published 18 articles. Nine institutions have published more than 10 articles.
| Institution name | Year | Centrality | Number of articles |
|---|---|---|---|
| UTMD Anderson Cancer Center | 2007 | 0.06 | 24 |
| Harvard University | 2011 | 0.08 | 20 |
| Institut National de la Sante et de la Recherche Medicale (Inserm) | 2012 | 0.07 | 18 |
| UDICE-French Research Universities | 2012 | 0 | 14 |
| Memorial Sloan Kettering Cancer Center | 2010 | 0.05 | 14 |
| Assistance Publique Hopitaux Paris (APHP) | 2012 | 0.04 | 13 |
| Fred Hutchinson Cancer Center | 2004 | 0.07 | 11 |
| Mayo Clinic | 2005 | 0.05 | 11 |
| Dana-Farber Cancer Institute | 2013 | 0.03 | 11 |
| Cornell University | 2010 | 0.01 | 10 |

-
Display of collaborative network of relevant literature research institutions.
Analysis of citations to the literature
As shown in Table 4 and Figure 5, Benjamin J. Chen; Bjoern Chapuy; Jing Ouyang, et al, published in CLIN CANCER RES/CLINICAL CANCER RESEARCH, PD-L1 Expression Is Characteristic of a Subset of PD-L1 Expression Is Characteristic of a Subset of Aggressive B-cell Lymphomas and Virus-Associated Malignancies is one of the most influential studies in the field of immunotherapy for DLBCL. There are two papers with more than 10 citations in the field of immunotherapy for DLBCL. Thirteen papers were cited more than five times. Seven papers were cited five times and ten papers were cited four times. Fourteen papers were cited three times. See Table 3 for information on authors, journals, and literature with more than six citations. Among the journals with more than six citations BLOOD appeared three times, the highest number. CLIN CANCER RES, NEW ENGL J MED, and J CLIN ONCOL appeared two times each. LANCET ONCOL, NATURE, and CLIN ONCOL appeared one time each.
| Highly cited scholar | Name of journal | DOI number | Number of citations | Centrality | Year |
|---|---|---|---|---|---|
| Chen BJ[30] | CLIN CANCER RES | 10.1158/1078-0432.CCR-13-0855 | 12 | 0.26 | 2013 |
| Ansell SM[31] | NEW ENGL J MED | 10.1056/NEJMoa1411087 | 11 | 0.05 | 2015 |
| Coiffier B[32] | NEW ENGL J MED | 10.1056/NEJMoa011795 | 10 | 0.19 | 2002 |
| Armand P[33] | J CLIN ONCOL | 10.1200/JCO.2012.48.3685 | 10 | 0.3 | 2013 |
| Cheson BD[34] | J CLIN ONCOL | 10.1200/JCO.2006.09.2403 | 9 | 0.18 | 2007 |
| Pfreundschuh M[35] | LANCET ONCOL | 10.1016/S1470-2045(06)70664-7 | 8 | 0.09 | 2006 |
| Kiyasu J[36] | BLOOD | 10.1182/blood-2015-02-629600 | 7 | 0.02 | 2015 |
| Herbst RS[37] | NATURE | 10.1038/nature14011 | 7 | 0 | 2014 |
| Swerdlow SH[38] | BLOOD | 10.1182/blood-2016-01-643569 | 7 | 0.02 | 2016 |
| Feugier P[39] | CLIN ONCOL | 10.1200/JCO.2005.09.131 | 7 | 0.26 | 2005 |
| Coiffier B[40] | BLOOD | 10.1182/blood-2010-03-276246 | 7 | 0.4 | 2010 |
| Andorsky DJ[41] | CLIN CANCER RES | 10.1158/1078-0432.CCR-10-2660 | 7 | 0.01 | 2011 |
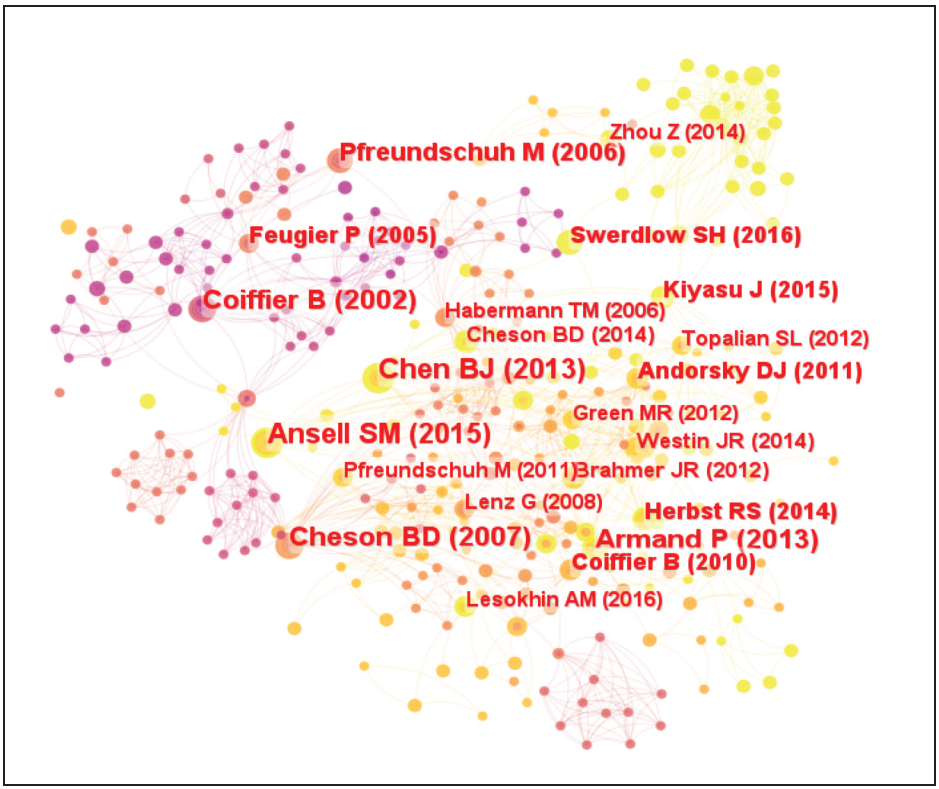
-
Network diagram of citation information.
Keyword analysis
Keyword co-occurrence analysis
Keywords are a high level summary of the main content of a piece of literature and can therefore be analyzed to examine topical issues in a particular field. Figure 6 shows the keyword co-occurrence mapping of the literature related to immunotherapy for DLBCL, obtained by co-occurrence analysis of keywords in the literature through CiteSpace. There are a total of 422 nodes and 2447 linked lines in the graph. Each node represents a keyword, with larger nodes representing more frequent occurrences of the keyword, and lines between nodes representing the degree of interconnectedness. The top three keywords were “expression,” “diffuse large b-cell lymphoma,” and “rituximab.” The top 20 keywords were disease names: “diffuse large B-cell lymphoma,” “non-Hodgkin’s lymphoma,” “B-cell lymphoma,” “follicular lymphoma,” and four others. The keywords related to treatment methods were: “rituximab,” “chemotherapy,” “immunotherapy,” “cancer immunotherapy,” and “transplantation” [Table 5].
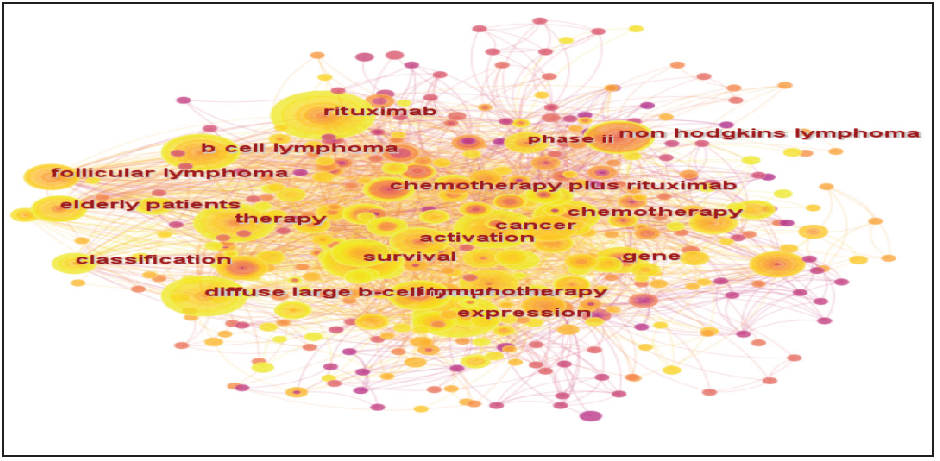
-
Keyword co-linear mapping of related literature.
| Keyword | Frequency | Year | Centrality | Keyword | Frequency | Year | Centrality |
|---|---|---|---|---|---|---|---|
| expression | 71 | 2004 | 0.17 | follicular lymphoma | 29 | 2004 | 0.13 |
| diffuse large B-cell lymphoma | 68 | 2005 | 0.09 | elderly patients | 27 | 2007 | 0.07 |
| rituximab | 57 | 2009 | 0.11 | classification | 21 | 2004 | 0.09 |
| therapy | 47 | 2003 | 0.07 | chop | 21 | 2008 | 0.03 |
| survival | 46 | 2009 | 0.11 | phase ii | 19 | 2003 | 0.06 |
| cancer | 46 | 2012 | 0.09 | outcome | 18 | 2017 | 0.03 |
| chemotherapy | 43 | 2003 | 0.18 | cancer immunotherapy | 16 | 2011 | 0.05 |
| non-Hodgkin’s lymphoma | 43 | 2003 | 0.13 | activation | 16 | 2009 | 0.05 |
| B-cell lymphoma | 42 | 2006 | 0.12 | transplantation | 16 | 2017 | 0.03 |
| immunotherapy | 32 | 2004 | 0.14 | gene | 15 | 2004 | 0.07 |
| Top terms | Size | Silhouette | Mean |
|---|---|---|---|
| pd-l1 (18.94, 1.0E-4); immune checkpoint (12.6, 0.001); expression (11.27, 0.001); survival (9.48, 0.005); pd 1 blockade (9.44, 0.005) | 77 | 0.694 | 2014 |
| antibody-based immunotherapy (17.51, 1.0E-4); diffuse large B-cell lymphoma (15.59, 1.0E-4); lymphoma and Hodgkin’s disease (13.99, 0.001); chop (10.48, 0.005); chemotherapeutic approaches (10.48, 0.005) | 64 | 0.74 | 2013 |
| cd19 (13.88, 0.001); B cell (11.35, 0.001); cellular (7.56, 0.01); chimeric antigen (7.56, 0.01); loncastuximab tesirine (7.56, 0.01) | 53 | 0.713 | 2015 |
| c myc (13.68, 0.001); ct (9.1, 0.005); phase i (9.1, 0.005); gene expression (9.1, 0.005); flow cytometry (9.1, 0.005) | 46 | 0.768 | 2014 |
| serex (15.18, 1.0E-4); cancer testis antigen (10.11, 0.005); gene (7.36, 0.01); dendritic cells (6.45, 0.05); b-cell lymphoma (5.42, 0.05) | 44 | 0.897 | 2007 |
| follicular lymphoma (10.46, 0.005); bispecific antibody (7.9, 0.005); radioimmunotherapy (7.9, 0.005); low grade (7.9, 0.005); detude des lymphm (7.9, 0.005) | 44 | 0.843 | 2008 |
| aggressive non-Hodgkin’s lymphoma (10.49, 0.005); immunohistochemistry (5.25, 0.05); alk plus large B cell lymphoma (5.23, 0.05); treatments (5.23, 0.05); bcl 6 protein expression (5.23, 0.05) | 37 | 0.702 | 2011 |
| aggressive lymphoma (10.46, 0.005); autologous transplantation (7.66, 0.01); hematopoietic stem cell transplantation (7.08, 0.01); ctl019 chimeric antigen receptor (7.08, 0.01); autologous stem cell transplantation (7.08, 0.01) | 24 | 0.872 | 2008 |
| cancer immunotherapy (20.69, 1.0E-4); antibody (15.12, 0.001); oncogenic stat3 signaling (12.99, 0.001); lymphoma microenvironment (12.99, 0.001); dlbcl mouse models (12.99, 0.001) | 23 | 0.863 | 2016 |
Keyword emergence analysis
A keyword emergent analysis can reflect the hot topics of research in a particular field at a particular time. A keyword emergent analysis yielded a graph of emergent terms in the field of immunotherapy for DLBCL [Figure 7]. “Bone marrow transplantation” was the earliest and longest-running term, appearing from 2004 to 2015.” “Immune response” was also the earliest, appearing from 2004 to 2010. The three keywords that appeared until 2022, the last year of inclusion, were “cancer immunotherapy,” “resistance,” and “cytokine release syndrome,” suggesting that this is a hot area of current research.

-
Analysis of keyword prominence in related literature.
Keyword clustering analysis
The keywords were analyzed by Log-Likelihood Ratio (LLR) algorithm, and the keywords in the field of immunotherapy for diffuse large B-cell lymphoma were divided into nine clusters [Figure 8 and Table 6]. The keyword clustering map is shown below. Several types of clusters were formed, with #0 being the largest, #1 the second largest, and so on. Among them “pd-l1,” “antibody-based,” “immunotherapy,” and “cd19” clusters are predominantly studied. As can be seen from the keyword clustering map in Figure 6, the clusters cover each other, indicating that the clusters are closely linked and the research topics have a concentrated character.
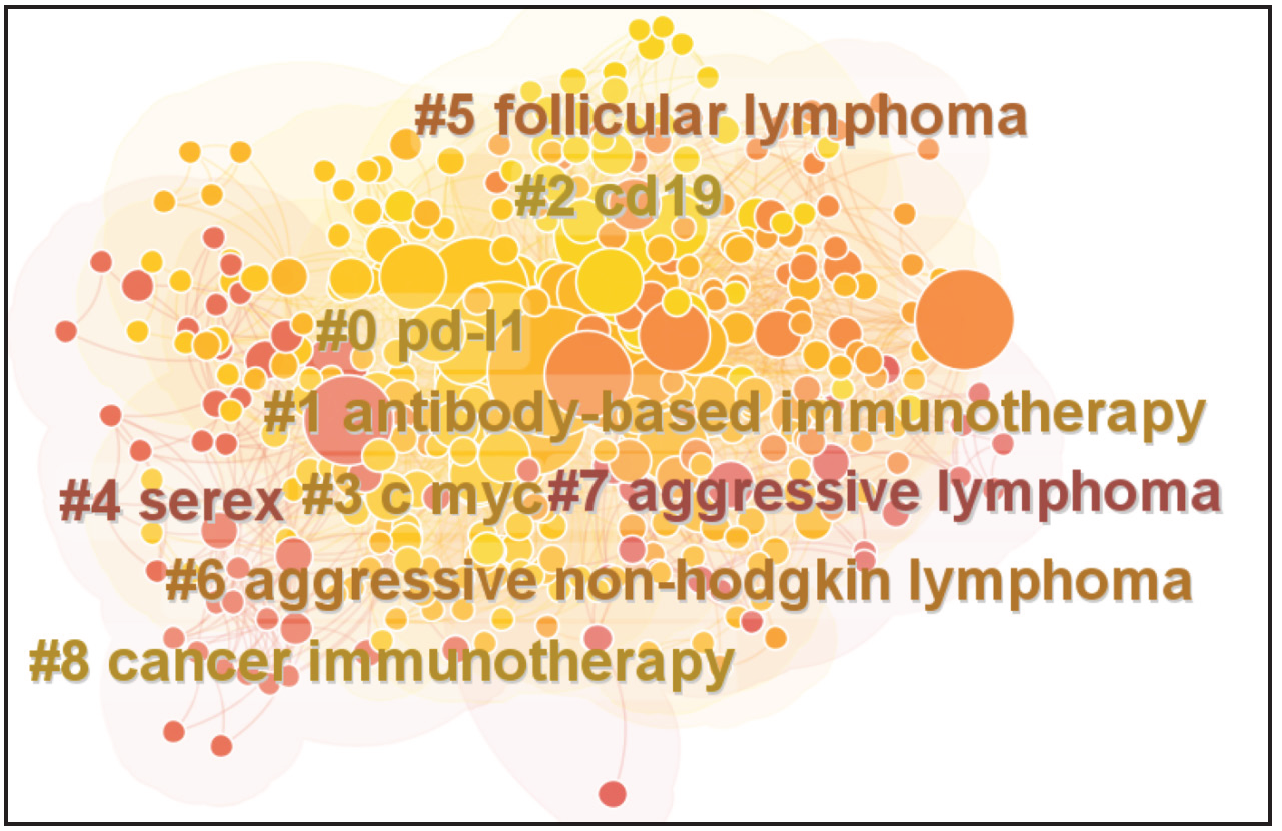
- Cluster analysis of keywords in related literature.
Discussion
As can be seen from the study results, the research on DLBCL immunotherapy has been on the rise over the past 20 years. Although the initial research literature is small and the related research results are limited, the research in this field has shown a clear upward trend in recent years. This indicates that the research in the field of DLBCL immunotherapy is gradually receiving more attention, and there is great potential for future research development.
International Research Gaps: The study found that the United States leads the way in DLBCL immunotherapy research, with more than twice as many articles as other countries. In contrast, other developing countries except China have less research on immunotherapy for DLBCL. This means that other countries’ research in this field needs to be further upgraded and improved to narrow the gap with leading countries.
Collaboration and interinstitutional collaboration: The findings show that the field of DLBCL immunotherapy involves multiple research institutions, and there is a strong collaboration between these institutions. In particular, UTMD Anderson Cancer Center, Harvard University, and French National Institute of Health and Medical Research. Many research articles have been published by institutions such as Inserm. This suggests that cross-institutional collaboration and collaboration in DLBCL’s research contributes to more impactful research outcomes. Therefore, future research can continue to encourage collaboration between institutions to advance the field.
Hot research areas: Keyword analysis showed that the hot research areas mainly focused on the expression of DLBCL, Rituximab and other treatment methods. In addition, treatment methods such as immunotherapy, chemotherapy, cancer immunotherapy and transplantation are also receiving attention. Future research could further explore these hot areas and further study them.
CONCLUSIONS
DLBCL is a malignant B-cell lymphoma and is one of the most common types of NHL.[1] Traditional treatments include radiotherapy and chemotherapy, but these treatments can cause many side effects and are not effective for some patients.[12,13] Immunotherapy is a treatment that targets the tumor’s immune system and can kill cancer cells by activating or boosting the body’s immune system. For patients with DLBCL, immunotherapy may involve using specific antibodies to recognize and attack cancer cells, or by transferring immune cells to attack cancer cells.[17,18]
Conducting a bibliometric study can help us better understand the literature on immunotherapy in the treatment of DLBCL in order to understand current research trends and hot spots in the field. This information can provide an important reference for clinicians or researchers to help them produce more impactful research in this area and provide valuable references and insights for future relevant research. In addition, bibliometric studies can help us understand the research hotspots and knowledge gaps in the field, and provide directions and recommendations for future relevant research.
Declaration of patients consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- CD19-Targeted immunotherapies for diffuse large B-cell lymphoma. Front Immunol. 2022;13:837457.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extranodal diffuse large B cell lymphoma: Molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. 2018;19:38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37:551-68.e14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020;13:175.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel Therapies for relapsed or refractory diffuse large B-cell lymphoma. Int J Mol Sci. 2020;21:8553.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- All roads lead to targeted diffuse large B-cell lymphoma approaches. Cancer Cell. 2022;40:131-3.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting B-cell receptor and PI3K signaling in diffuse large B-cell lymphoma. Blood. 2021;138:1110-9.
- [CrossRef] [PubMed] [Google Scholar]
- All roads lead to targeted diffuse large B-cell lymphoma approaches. Cancer Cell. 2022;40:131-3.
- [CrossRef] [PubMed] [Google Scholar]
- The immunology of DLBCL. Cancers (Basel). 2023;15:835.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ROBUST: A phase III study of Lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021;39:1317-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tafasitamab for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther. 2021;21:455-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94:604-16.
- [CrossRef] [PubMed] [Google Scholar]
- Extranodal diffuse large B cell lymphoma: Molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. 2018;19:38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell. 2021;39:1643-53.e3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37:551-68.e14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Delivering safer immunotherapies for cancer. Adv Drug Deliv Rev. 2017;114:79-101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86-104.
- [CrossRef] [PubMed] [Google Scholar]
- Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 2018;8:180037.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open. 2019;2:e1918007.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Bibliometric and visual analysis of global community Resilience Research. Int J Environ Res Public Health. 2021;18:10857.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Schizophrenia and inflammation research: A bibliometric analysis. Front Immunol. 2022;13:907851.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bibliometric analysis of neurology articles published in general medicine journals. JAMA Netw Open. 2021;4:e215840.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Knowledge domain and emerging trends in Alzheimer’s disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. 2019;14:1643-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Study on pain catastrophizing from 2010 to 2020: A bibliometric analysis via CiteSpace. Front Psychol. 2021;12:759347.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bibliometric analysis of bronchopulmonary dysplasia in extremely premature infants in the web of science database using CiteSpace software. Front Pediatr. 2021;9:705033.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A CiteSpace-Based Analysis of the Development Trends Affecting Clinical Research Nurses in China: A Systematic Review. J Multidiscip Healthc. 2022;15:2363-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-42.
- [CrossRef] [PubMed] [Google Scholar]
- Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199-206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-86.
- [CrossRef] [PubMed] [Google Scholar]
- CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006 May;7:379-91.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193-201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117-26.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232-44.
- [CrossRef] [PubMed] [Google Scholar]







