Translate this page into:
Impact of shifting of hormonal receptor and human epidermal growth factor receptor 2 on survival of non-metastatic breast cancer
* Corresponding author: Dr. Sanskriti Poddar, MD, DNB, Department of Radiotherapy, Serampore Walsh Sub Divisional Hospital, Hoogly, Serampore, West Bengal, India. sanskritipoddar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh D, Chandra B, Maiti Das S, Poddar S, Dasgupta P, Das S. Impact of shifting of hormonal receptor and human epidermal growth factor receptor 2 on survival of non-metastatic breast cancer. Asian J Oncol. 2025;11:7. doi: 10.25259/ASJO_41_2024
Abstract
Objectives
This study explores variations in hormone receptor (HR) status between biopsy and post-operative reports, as well as changes following neoadjuvant chemotherapy (NACT). The correlation between HR status alterations and patient survival outcomes is the key focus of this investigation.
Material and Methods
This retrospective study conducted at a tertiary care center in Kolkata, Eastern India, from 2013 to 2018.
Results
The parameters of 482 breast cancer patients meeting inclusion criteria were studied. The majority were >40 years (68.2%), with 300 receiving NACT. Clinical staging distribution was I (2.9%), IIA (10.4%), IIB (22.2%), IIIA (34.3%), IIIB (28.6%), and IIIC (1.6%). Preoperative and postoperative staging changes occurred in 47.7%, with 38.8% downstaging and 13.5% upstaging. Estrogen receptor (ER), progesterone receptor (PR), and Her2neu positivity at diagnosis were observed in 46.3%, 41.9%, and 36.9% of patients, respectively. Postoperative ER, PR, and Her2neu positivity were 46.1%, 40%, and 37.3%, respectively. Recurrence in 33.6% of patients correlated with factors like age, tumor grade, lymphovascular invasion (LVI), Perineural invasion (PNI), postoperative stage, ER, PR, and their changes. Multivariate analysis identified age, PNI, postoperative stage, stage change, ER, and PR changes as independent factors for recurrence. The correlation study demonstrated a significant association between NACT and changes in PR status (χ2 = 16.56; p = 0.001), while no significant associations were found for ER and Her2neu changes. Analysis of variance (ANOVA) revealed a substantial association between NACT and PR status changes (p = 0.001). Post Hoc tests indicated significant differences in PR status changes related to NACT. Kaplan-Meier survival analysis revealed a significant difference in disease-free survival (DFS) based on changes in ER and PR status, with ER-positive patients having a median DFS of 76 months and PR-positive patients showing a median DFS of 47 months. No significant DFS difference was observed for changes in Her2neu status.
Conclusion
Changes in hormone receptor status, particularly ER and PR, significantly impacted recurrence and DFS. The study highlights the importance of personalized management strategies, with age, tumor grade, lymphovascular invasion (LVI), and changes in ER and PR status identified as key factors influencing prognosis.
Keywords
Breast cancer
Estrogen and progesterone receptor
Neoadjuvant chemotherapy
Hormonal receptor change
Survival
INTRODUCTION
The number of new breast cancer cases in India is 178,361 (13.5%), with 90,408 (10.6%) reported deaths, as per the global cancer observatory (GLOBOCAN) data (2020).[1] The role of neoadjuvant chemotherapy (NACT) is now established for the treatment of locally advanced breast cancer. It helps to reduce the tumor size and, most importantly, decrease micrometastasis, increasing the chances of breast-conserving surgery.[2,3] The receptor’s status needs to be determined as per protocol before initiating the treatment of breast cancer. These receptors include estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 neu (Her2neu), and Ki 67%. Hormone receptors (HR) and molecular markers are used to determine the status of breast cancer, and they may change after neoadjuvant chemotherapy.[4] This retrospective study seeks to illustrate the variations in HR status between biopsy specimens and final histopathological reports post-operatively, along with assessing changes in HR status following NACT at a tertiary care center in Eastern India. Additionally, the study aims to establish a correlation between the alterations in HR status and the survival outcomes of the patients.
MATERIAL AND METHODS
The clinical data of breast cancer patients treated at the Institute of Post Graduate Medical Education and Research (IPGMER), Kolkata, from January 2013 to December 2018 were collected. The staging was done based on the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging system (7th edition, 2010).[5] The diagnosis was validated by histopathological examination. The inclusion criteria for the patients were as follows: age > 18 years; females; unilateral histopathologically confirmed invasive breast carcinoma; availability of pre and post-operative histopathological examination report including the immunohistochemistry (IHC) report of ER, PR, Her2neu, and Ki67%; nonmetastatic breast carcinoma. Exclusion criteria were as follows: metastatic breast cancer; non-availability of pre and post-operative IHC report of ER, PR, Her2neu, and Ki67%; previous history of breast cancer treatment.
Data on age group, menopausal status, histopathological status, clinical stage, surgical information, post-operative staging, systemic chemotherapy information, IHC status, and follow-ups was collected. Disease-free survival (DFS) was defined from the start of primary therapy to the date of disease recurrence (local or distant). Overall survival (OS) was defined as the time from the date of the start of primary therapy to the date of death or the last follow-up.
Statistical analysis
IBM SPSS (Statistical Package for the Social Sciences) for Windows version 25.0 was used.[6] Descriptive statistics were used to characterize the patient population using frequencies, mean, median, and quartile values. Continuous variables were analyzed using the Student’s t-test. Univariate and multivariate Cox regression analyses were performed to identify the factors associated with disease recurrence. Analysis of variance (ANOVA) test done to identify the pre and post-NACT receptor status changes. DFS and OS were analyzed with the Kaplan–Meier test, and survival curves were compared with the log-rank test. P-values <0.05 were accepted as significant.
RESULTS
Patients meeting the inclusion criteria were selected for analysis. The majority of the patients were in the age group > 40 years (68.2%). NACT was received by 300 patients whereas 182 were considered for upfront surgical intervention.
Clinicopathological characteristics
The distribution of clinical staging of patients at stage I, IIA, IIB, IIIA, IIIB, and IIIC was 2.9%, 10.4%, 22.2%, 34.3%, 28.6%, and 1.6% respectively. The ER, PR, and Her2neu were positive in 46.3%, 41.9%, and 36.9% of patients respectively, at diagnosis of the core needle biopsy specimen. The distribution of grades of the tumor at grade 1, 2, and 3 were 20.5%, 40.5%, and 39%, respectively.
The post-operative stages IA, IIA, IIB, IIIA, IIIB, and IIIC were distributed as 5%, 19.5%, 30.5%, 25.3%, 13.1%, and 6.6%, respectively. The comparison between pre-and post-operative staging revealed that there was no change in 47.7% of patients. Down-staging was observed in 38.8% of patients, and up-staging was seen in 13.5%. The IHC study on the post-operative specimen revealed that the ER, PR, and Her2neu were positive in 46.1%, 40%, and 37.3% of patients, respectively.
The analysis of the pattern of changes in the ER of the post-operative specimen showed that the status remained positive for 41.9% of patients. and negative in 49.6%. ER from positive to negative was observed in 4.4%, and from negative to positive change in 4.1% of patients. The PR change pattern in the post-operative specimen showed that 35.7% remained positive and 53.7% remained negative. The change of PR from positive to negative and from negative to positive were 6.2% and 4.4%, respectively. Similarly, the Her2neu status of the post-operative specimen remained positive and negative in 34.4% and 60.2% of patients, respectively. The change of Her2neu from positive to negative and from negative to positive was in 2.5% and 2.9% of patients, respectively, in the post-operative specimen. At the time of analysis, 162 (33.6%) of the patients had recurrence. The comparative clinicopathological characteristics have been given in Table 1.
| Variable | Characteristics | Neoadjuvant chemotherapy (NACT) | P - value | |||
|---|---|---|---|---|---|---|
| No (N =182) | Yes (N =300) | |||||
| Count | N % | Count | N % | |||
| Side | Left | 91 | 18.88% | 165 | 34.24% | 0.286 |
| Right | 91 | 18.88% | 135 | 28.00% | ||
| Age group | ≤ 40 years | 60 | 12.44% | 93 | 19.30% | 0.653 |
| > 40 years | 122 | 25.35% | 207 | 42.91% | ||
| Clinical T | T 1 | 20 | 4.14% | 0 | 0.00% | <0.001 |
| T 2 | 112 | 23.24% | 20 | 4.14% | ||
| T 3 | 39 | 8.10% | 146 | 30.30% | ||
| T 4 | 11 | 2.28% | 134 | 27.80% | ||
| Clinical N | N 0 | 75 | 15.57% | 24 | 4.97%% | <0.001 |
| N 1 | 97 | 20.13% | 184 | 38.18% | ||
| N 2 | 10 | 2.07% | 84 | 17.43% | ||
| N 3 | 0 | 0.00% | 8 | 1.65% | ||
| Clinical stage | IA | 14 | 2.90% | 0 | 0.00% | <0.001 |
| IIA | 48 | 9.96% | 2 | 0.42% | ||
| IIB | 83 | 17.22% | 24 | 4.98% | ||
| IIIA | 26 | 5.40% | 139 | 28.81% | ||
| IIIB | 11 | 2.28% | 127 | 26.32% | ||
| IIIC | 0 | 0.00% | 8 | 1.66% | ||
| ER (Biopsy specimen) | Negative | 93 | 19.30% | 166 | 34.44% | 0.366 |
| Positive | 89 | 18.46% | 134 | 27.80% | ||
| PR (Biopsy specimen) | Negative | 102 | 21.16% | 178 | 36.93% | 0.478 |
| Positive | 80 | 16.60% | 122 | 25.31% | ||
| Her2neu (Biopsy specimen) | Negative | 105 | 21.79% | 199 | 41,29% | 0.057 |
| Positive | 77 | 15.97% | 101 | 20.95% | ||
| Pathological T | T 1 | 22 | 4.56% | 8 | 1.65% | <0.001 |
| T 2 | 125 | 25.95% | 92 | 19.09% | ||
| T 3 | 24 | 4.97% | 136 | 28.23% | ||
| T 4 | 11 | 2.28% | 64 | 13.27% | ||
| Pathological N | N 0 | 80 | 16.60% | 120 | 24.90% | 0.026 |
| N 1 | 62 | 12.86% | 90 | 18.68% | ||
| N 2 | 36 | 7.46% | 63 | 13.07% | ||
| N 3 | 4 | 0.83% | 27 | 5.60% | ||
| Post-operative stage | IA | 17 | 3.52% | 7 | 1.48% | <0.001 |
| IIA | 63 | 13.08% | 31 | 6.42% | ||
| IIB | 48 | 9.96% | 99 | 20.54% | ||
| IIIA | 40 | 8.29% | 82 | 17.01% | ||
| IIIB | 9 | 1.86% | 54 | 11.24% | ||
| IIIC | 5 | 1.04% | 27 | 5.56% | ||
| Change of stage | Stable | 119 | 24.70% | 111 | 23.02% | <0.001 |
| Upstaged | 31 | 6.45% | 34 | 7.05% | ||
| Downstaged | 32 | 6.64% | 155 | 32.16% | ||
| Post-operative ER | Negative | 94 | 19.50% | 166 | 34.50% | 0.431 |
| Positive | 88 | 18.25% | 134 | 27.85% | ||
| Post-operative PR | Negative | 101 | 21.00% | 188 | 39.00% | 0.119 |
| Positive | 81 | 16.80% | 112 | 23.20% | ||
| Post-operative Her2neu | Negative | 105 | 21.79% | 197 | 40.88% | 0.079 |
| Positive | 77 | 15.97% | 103 | 21.36% | ||
| Change of ER | Stable positive | 84 | 17.42% | 118 | 24.48% | 0.127 |
| Positive to negative | 5 | 1.06% | 16 | 3.31% | ||
| Negative to positive | 4 | 0.82% | 16 | 3.31% | ||
| Stable negative | 89 | 18.47% | 150 | 31.13% | ||
| Change of PR | Stable positive | 77 | 15.99% | 95 | 19.71% | 0.001 |
| Positive to negative | 3 | 0.63% | 27 | 5.60% | ||
| Negative to positive | 4 | 0.82% | 17 | 3.52% | ||
| Stable negative | 98 | 20.33% | 161 | 33.40% | ||
| Change of Her2neu | Stable positive | 74 | 15.35% | 92 | 19.08% | 0.088 |
| Positive to negative | 3 | 0.62% | 9 | 1.87% | ||
| Negative to positive | 3 | 0.62% | 11 | 2.28% | ||
| Stable negative | 102 | 21.17% | 188 | 39.01% | ||
| LVI | Negative | 81 | 16.80% | 110 | 22.82% | 0.088 |
| Positive | 101 | 20.96% | 190 | 39.42% | ||
| PNI | Negative | 90 | 18.67% | 114 | 23.66% | 0.014 |
| Positive | 92 | 19.08% | 186 | 38.59% | ||
| Tumor grade | Grade 1 | 59 | 12.24% | 40 | 8.30% | <0.001 |
| Grade 2 | 70 | 14.52% | 125 | 25.94% | ||
| Grade 3 | 53 | 11.00% | 135 | 28.00% | ||
| Recurrence | Yes | 52 | 10.78% | 110 | 22.82% | 0.068 |
| No | 130 | 26.98% | 190 | 39.42% | ||
LVI: Lymphovascular invasion, PNI: Perineural invasion, ER: Estrogen receptor, PR: Progesterone receptor, T: Tumor, N: Node.
Univariate and multivariate Cox regression analysis
The Cox regression analysis was performed to identify the factors associated with recurrence; details have been given in Table 2. The univariate Cox regression analysis showed that age group (p = 0.043), tumor grade (p = 0.002), LVI (p < 0.001), PNI (p < 0.001), post-operative stage p < 0.001), change of stage (p = 0.037), post-operative ER status (p < 0.001), post-operative PR status (p < 0.001), change of ER status (p < 0.001), change of PR status (p < 0.001) were dependent factors for recurrence in breast cancer patients. Menopausal status (p = 0.508), NACT (p = 0.114), and change of Her2neu (p = 0.245) were not associated with recurrence. The multivariate Cox regression analysis of dependent factors showed that age group (p < 0.001), PNI (p = 0.015), post-operative stage (p < 0.001), change of stage (p = 0.041), post-operative ER status (p = 0.013), change of PR status (p = 0.030) were the independent factors associated with recurrence in breast cancer patients.
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| N | P value | HR | 95% CI for HR | P value | HR | 95% CI for HR | ||
| Age group | 0.043 | 0.001 | ||||||
| ≤ 40 years | 153 | 1 | 1 | |||||
| > 40 years | 329 | 0.71 | 0.52 – 0.99 | 0.57 | 0.41 – 0.80 | |||
| Menopausal status | 0.508 | |||||||
| Pre-menopausal | 242 | 1 | ||||||
| Post-menopausal | 240 | 0.90 | 0.66 – 1.22 | |||||
| Neoadjuvant chemotherapy | 0.114 | |||||||
| No | 182 | 1 | ||||||
| Yes | 300 | 1.30 | 0.93 – 1.81 | |||||
| Grade | 0.002 | 0.669 | ||||||
| Grade 1 | 99 | 1 | 1 | |||||
| Grade 2 | 195 | 0.021 | 1.77 | 1.08 – 2.87 | 0.427 | 1.23 | 0.73 – 2.08 | |
| Grade 3 | 188 | 0.001 | 2.31 | 1.43 – 3.72 | 0.378 | 1.26 | 0.74 – 2.15 | |
| LVI | <0.001 | 0.227 | ||||||
| Absent | 191 | 1 | 1 | |||||
| Present | 291 | 1.99 | 1.41 – 2.80 | 1.25 | 0.86 – 1.82 | |||
| PNI | <0.001 | 0.015 | ||||||
| Absent | 204 | 1 | 1 | |||||
| Present | 278 | 2.56 | 1.80 – 3.65 | 1.60 | 1.09 – 2.35 | |||
| Post-operative stage | <0.001 | <0.001 | ||||||
| Stage IA | 24 | 1 | 1 | |||||
| Stage IIA | 94 | 0.742 | 1.19 | 0.42 – 3.34 | 0.559 | 0.72 | 0.24 – 2.12 | |
| Stage IIB | 147 | 0.72 | 2.34 | 0.92 – 5.95 | 0.385 | 1.55 | 0.57 – 4.22 | |
| Stage IIIA | 122 | 0.004 | 3.95 | 1.54 – 10.14 | 0.029 | 3.13 | 1.12 – 8.70 | |
| Stage IIIB | 63 | 0.001 | 5.35 | 2.06 – 13.89 | 0.018 | 3.50 | 1.24 – 9.58 | |
| Stage IIIC | 32 | <0.001 | 7.784 | 2.89 – 20.90 | <0.001 | 13.34 | 3.39 – 52.42 | |
| Change of stage | 0.037 | 0.041 | ||||||
| Stable stage | 230 | 1 | 1 | |||||
| Upstaged | 65 | 0.478 | 1.173 | 0.75 - 1.82 | 0.016 | 0.35 | 0.15 – 0.82 | |
| Downstaged | 187 | 0.028 | 0.679 | 0.48 – 0.95 | 0.647 | 1.10 | 0.718 – 1.70 | |
| Post-operative ER | <0.001 | 0.013 | ||||||
| Negative | 260 | 1 | 1 | |||||
| Positive | 222 | 0.460 | 0.33 – 0.64 | 0.49 | 0.28 – 0.86 | |||
| Post-operative PR | <0.001 | 0.737 | ||||||
| Negative | 289 | 1 | 1 | |||||
| Positive | 193 | 0.520 | 0.37 – 0.72 | 0.90 | 0.51 – 1.59 | |||
| Post operative Her2neu | 0.510 | |||||||
| Negative | 302 | 1 | ||||||
| Positive | 180 | 0.897 | 0.64 – 1.24 | |||||
| Change of ER | <0.001 | 0.074 | ||||||
| Positive to positive | 202 | 1 | 1 | |||||
| Positive to negative | 21 | 0.238 | 1.545 | 0.75 - 3.18 | 0.211 | 1.61 | 0.76 – 3.42 | |
| Negative to positive | 20 | 0.342 | 0.609 | 0.21 – 1.69 | 0.605 | 1.38 | 0.40 – 4.76 | |
| Negative to negative | 239 | <0.001 | 2.134 | 1.50 – 3.01 | 0.013 | 2.03 | 1.15 – 3.56 | |
| Change of PR | <0.001 | 0.030 | ||||||
| Positive to positive | 172 | 1 | 1 | |||||
| Positive to negative | 30 | 0.789 | 0.902 | 0.42 – 1.91 | 0.368 | 0.69 | 0.32 – 1.52 | |
| Negative to positive | 21 | 0.087 | 0.359 | 0.11 – 1.16 | 0.025 | 0.20 | 0.05 – 0.81 | |
| Negative to negative | 259 | 1.870 | 1.870 | 1.32 – 2.64 | 0.737 | 1.10 | 0.62 – 1.93 | |
| Change of Her2neu | 0.245 | |||||||
| Positive to positive | 166 | 1 | ||||||
| Positive to negative | 12 | 0.275 | 0.567 | 0.20 – 1.57 | ||||
| Negative to positive | 14 | 0.129 | 0.335 | 0.08 – 1.37 | ||||
| Negative to negative | 290 | 0.663 | 1.076 | 0.77 – 1.47 | ||||
ER: Estrogen receptor, PR: Progesterone receptor, LVI:Lymphovascular invasion, PNI: Perineural invasion, HR: Hazard ratio, CI: Confidence interval.
Correlation study of change of receptor status and NACT
The correlation study showed that a change in PR status (χ2 = 16.56; p = 0.001) was statistically significantly associated with NACT. Change of ER status (χ2 = 5.70; p = 0.127) and Her2neu (χ2 = 6.53; p = 0.089) were statistically significantly not associated with NACT. ANOVA between NACT and change of ER, PR, and Her2neu status of the post-operative specimen showed that change of PR status is statistically significantly associated with NACT (p = 0.001); details have been given in Table 3. The post Hoc test between NACT and change of PR showed that there is a statistically significant difference between change from positive to positive and positive to negative (p < 0.001); positive to positive and negative to positive (p = 0.020); positive to negative and negative to negative (p = 0.003); details have been given in Table 4. It can be said that there is maximum change in PR status in association with NACT is observed in the group of patients who showed change from positive to negative.
| NACT and Change of ER | |||||
|---|---|---|---|---|---|
| Receptor change | N | Mean | Std. Deviation | 95% Confidence Interval | P value |
| Positive to positive | 202 | 1.58 | .494 | 1.52 - 1.65 | 0.127 |
| Positive to negative | 21 | 1.76 | .436 | 1.56 - 1.96 | |
| Negative to positive | 20 | 1.80 | .410 | 1.61 - 1.99 | |
| Negative to negative | 239 | 1.63 | .484 | 1.57 - 1.69 | |
| NACT and Change of PR | |||||
| Positive to positive | 172 | 1.55 | 0.499 | 1.48 - 1.63 | 0.001 |
| Positive to negative | 30 | 1.90 | 0.305 | 1.79 - 2.01 | |
| Negative to positive | 21 | 1.81 | 0.402 | 1.63 - 1.99 | |
| Negative to negative | 259 | 1.62 | 0.486 | 1.56 - 1.68 | |
| NACT and Change of Her2neu | |||||
| Positive to positive | 166 | 1.55 | 0.499 | 1.48 - 1.63 | 0.089 |
| Positive to negative | 12 | 1.75 | 0.452 | 1.46 - 2.04 | |
| Negative to positive | 14 | 1.79 | 0.426 | 1.54 - 2.03 | |
| Negative to negative | 290 | 1.65 | 0.478 | 1.59 - 1.70 | |
NACT: Neoadjuvant chemotherapy, ER: Estrogen receptor, PR: Progesterone receptor.
| (I) Change of PR | (J) Change of PR | Mean difference (I-J) | P value | 95% confidence interval |
|---|---|---|---|---|
| Positive to positive | Positive to negative | -0.348* | 0.000 | 0.53 - 0.16 |
| Negative to positive | -0.257* | 0.020 | 0.47 - 0.04 | |
| Negative to negative | -0.069 | 0.141 | 0.16 - 0.02 | |
| Positive to negative | Positive to positive | 0.348* | 0.000 | 0.16 - 0.53 |
| Negative to positive | 0.090 | 0.507 | 0.18 - 0.36 | |
| Negative to negative | 0.278* | 0.003 | 0.10 - 0.46 | |
| Negative to positive | Positive to positive | 0.257* | 0.020 | 0.04 - 0.47 |
| Positive to negative | -0.090 | 0.507 | 0.36 - 0.18 | |
| Negative to negative | 0.188 | 0.084 | 0.03 - 0.40 | |
| Negative to negative | Positive to positive | 0.069 | 0.141 | 0.02 - 0.16 |
| Positive to negative | -0.278* | 0.003 | 0.46 - 0.10 | |
| Negative to positive | -0.188 | 0.084 | 0.40 - 0.03 |
Survival analysis
The Kaplan-Meier survival analysis revealed a statistically significant difference in the DFS concerning changes in ER status (log-rank test, χ2 = 24.24; p < 0.001). The overall median DFS was 58.03 months. Patients maintaining ER positivity exhibited a median DFS of 76 months, while those remaining ER-negative had a median DFS of 35 months (CI of 29.56 – 40.43). ER changes from positive to negative resulted in a median DFS of 36 months (CI of 27.21 – 44.78), and DFS was not reached for ER changes from negative to positive [Figure 1]. Similarly, a significant difference in DFS was noted for changes in PR status (log-rank test, χ2 = 22.92; p < 0.001). PR-positive patients had a median DFS of 47 months (CI of 35.73–58.26), PR-negative patients showed a median DFS of 36 months (CI of 29.94–42.05), PR changes from positive to negative yielded a median DFS of 42 months (CI of 38.21–45.78), and those changing from PR negative to positive did not reach the median DFS [Figure 2]. No statistically significant difference in DFS was observed when comparing changes in Her2neu status
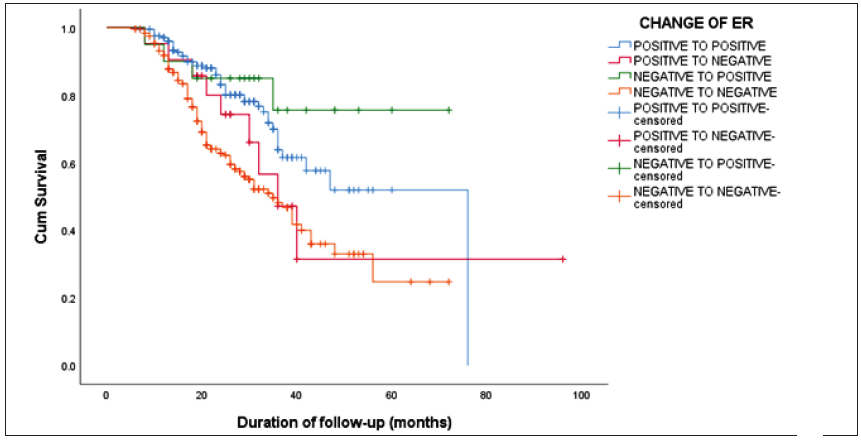
- The Kaplan-Meier survival curve showing a statistically significant difference in the disease-free survival (DFS) concerning changes in estrogen receptor (ER) status (log-rank test, χ2 = 24.24; p < 0.001).
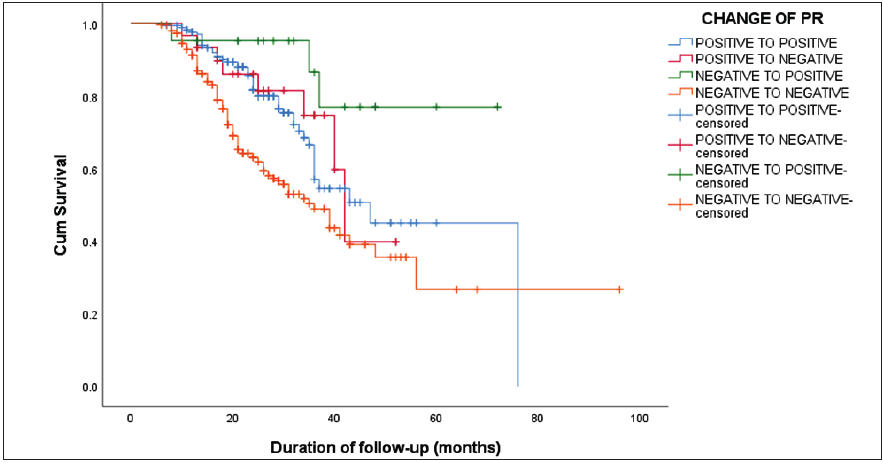
- The Kaplan-Meier survival curve showing a statistically significant difference in the disease-free survival (DFS) concerning changes in progesterone receptor (PR) status (log-rank test, χ2 = 22.92; p < 0.001).
DISCUSSION
This study mainly focuses on the change of ER and PR status in the specimens, but Her2neu status was also compared. Most important changes of receptor status are negative to positive as this change altered the treatment modality.[7] Our study revealed that ER and PR receptor status conversion or change influences the recurrence of breast cancer, and similar findings in the other studies showed that the ER and PR receptor changes influence survival.[8] In an Italian series of 904 patients, ER was lost in 5% of HR+ cancers compared with 67% showing loss of PR (cut-off 20%); change in PR status was associated with improved survival regardless of whether they received endocrine therapy.[9]The study found that change in PR receptor status is associated with improved survival which is in concordance with the findings of our study. A review of 45 studies addressing ER, PR, and Her2neu expression found an average prevalence of discordant results of 32% for PR and 13% for ER local recurrences showed higher rates of ER and PR conversion than the immediate post-NACT specimens.[10]
In our study, alterations in ER receptor status were observed in 8.5% (receptor changes of 1.88% in no NACT and 6.62% in the NACT group) of patients, while changes in PR receptor status were identified in 10.58% of patients. In a French series with 420 patients, ER status changed by 23%; 42% negative to positive and 13% positive to negative.[11] An Indian study by Anand AS et al. reported that ER discordance was 8.7 % and PR discordance was 13 %. PR positive to PR negative discordance was the predominant one. These findings were in concordance with the results of our study.[12] A study from China by WU YT et al. found a 15% change in ER status and 2a 7% change in PR status. These reports are higher than the findings of our study.[13] In this study, a statistically significant difference in patient DFS was observed based on changes in ER or PR status. Patients maintaining ER positivity exhibited a median survival of 76 months, while ER-negative patients had a median survival of 35 months. ER changes from positive to negative resulted in a median survival of 36 months, and for ER changes from negative to positive, the median survival was not attained. For PR, those remaining PR-negative had a median survival of 36 months, PR changes from positive to negative showed a median survival of 42 months, and those changing from PR-negative to positive did not reach the median survival. A study by Yang Z et al. reported findings similar to those of our study, except that their study involved metastatic breast cancers.[14]
A recent series of 482 patients by Ding et al. found changes in ER status in 10% of cases (36 positive to negative and 14 negative to positive) and PR status in 17% (57 positive to negative and 25 negative to positive). These changes influence the DFS and OS.[15] Yang L et al. described in their study that for patients who received adjuvant hormonal therapy after surgery, the 5-year DFS estimates for patients in any receptor conversion group (55.2%) was worse than patients in the receptor stable group (73.7%, Log-rank test, P = 0.015). While the 5-year OS estimates for patients with or without receptor conversion were not statistically different (86.0 vs. 82.4%, Log-rank test, P = 0.587).[16] We noted a lower survival rate in cases where ER expression was lost compared to those with continuous ER positivity. Likewise, DFS was compromised with the loss of PR expression and the shift from PR positive to PR negative. However, survival was higher in cases where PR initially tested positive but later converted to negative, in contrast to those where PR positivity was sustained. Matsumoto et al. found that patients exhibiting a switch to positive hormonal receptors in metastatic lesions experienced improved survival.[17] In accordance with the recent recommendations from the National Comprehensive Cancer Network (NCCN), it is advised to tailor treatment based on recurrent ER/PR/HER2 status whenever possible.[18] Ensure biopsy provision and optimize the treatment approach for patients experiencing recurrence and metastasis.
Limitations of our study including single center study, retrospective, and small sample size. Further studies on cumulative data are needed for confirmation.
CONCLUSION
In conclusion, changes in hormone receptor status, particularly ER and PR, significantly impacted recurrence and DFS. The study highlights the importance of personalized management strategies, with age, tumor grade, LVI, and changes in ER and PR status identified as key factors influencing prognosis. Further research is warranted to unravel the intricate relationships between molecular changes, treatment modalities, and long-term outcomes in breast cancer.
Acknowledgement
Department of Pathology, Institute of Post Graduate Medical Education & Research, Seth Sukhlal Karnani Memorial Hospital, Kolkata.
Author contribution
DS: Conceived & designed the analysis, contributed data or analysis tools, performed the analysis, manuscript editing, BC: Data collection, contributed data or analysis tools, wrote the paper, SMD: Conceived & designed the analysis, collected the data, manuscript editing, SP: Collected data, wrote the paper, manuscript editing. PD: Conceived & designed the analysis, manuscript editing, SD: Contributed data or analysis tools, manuscript editing.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Breast cancer in India: Present scenario and the challenges ahead. World J Clin Oncol. 2022;13:209-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J. Cancer Res. Clin. Oncol. 2023;149:1195-209.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of the axilla and the breast after neoadjuvant chemotherapy in patients with breast cancer: A systematic review. Sisli Etfal Hastan Tip Bul. 2021;55:156-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Study of ER, PR, HER2/neu, p53, and Ki67 expression in primary breast carcinomas and synchronous metastatic axillary lymph nodes. Indian J. Cancer. 2020;57:190-7.
- [CrossRef] [PubMed] [Google Scholar]
- The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471-4.
- [CrossRef] [PubMed] [Google Scholar]
- IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp; Released 2020.
- Receptor status after neoadjuvant therapy of breast cancer: Significance and implications. Pathobiology. 2022;89:297-308.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of neoadjuvant chemotherapy on estrogen and progesterone receptor expression and hormone receptor status in breast cancer. Am. J. Surg. 2003;186:348-50.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in pgR and ki-67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann. Oncol. 2015;26:307-13.
- [CrossRef] [PubMed] [Google Scholar]
- Variability in hormone and growth factor receptor expression in primary versus recurrent, metastatic, and post-neoadjuvant breast carcinoma. Breast Cancer Res. Treat. 2012;135:29-37.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2007;12:636-43.
- [CrossRef] [PubMed] [Google Scholar]
- Discordance of estrogen & progesterone receptors after neoadjuvant chemotherapy in breast cancer-An Indian study. Indian J Surg Oncol. 2016;7:316-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of neoadjuvant chemotherapy on the expression of hormone receptors and ki-67 in chinese breast cancer patients: A retrospective study of 525 patients. J Biomed Res. 2017;32:191-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The prognostic impact of hormonal receptor and HER-2 expression discordance in metastatic breast cancer patients. Onco Targets Ther. 2020;13:853-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact on survival of estrogen receptor, progesterone receptor and ki-67 expression discordance pre- and post-neoadjuvant chemotherapy in breast cancer. PLoS One. 2020;15:e0231895.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical significance and prognostic value of receptor conversion in hormone receptor positive breast cancers after neoadjuvant chemotherapy. World J. Surg. Oncol. 2018;16:51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic implications of receptor discordance between primary and recurrent breast cancer. Int. J. Clin. Oncol. 2015;20:701-8.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2022;20:691-722.
- [CrossRef] [PubMed] [Google Scholar]







