Translate this page into:
Dermatofibrosarcoma: Case Report Series
Address for correspondence Ida Bagus Made Suryawisesa, MD, MS, Department of General Surgery and Oncologic Surgery, Faculty of Medicine, Udayana University/Sanglah General Hospital, Denpasar, Pulau Serangan Street No. 5B, Denpasar, Bali 80114, Indonesia. gusdeck2001@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, painless, slow-growth, superficial soft tissue malignant sarcoma corresponding to less than 0.1% of all malignancies. The primary treatment for DFSP is surgical excision, which is wide local excision (WLE) with tumor-free margins, Mohs micrographic surgery (MMS), and partial or total amputation. The goal of surgical excision is to achieve negative resection margins, thus reducing the local recurrence rate. These three cases reported large dermatofibrosarcoma, which began as a small nodule and progressed within approximately a year and were treated subsequently with wide excisions surgery. The unique and challenging part for most surgeons is removing the mass with a concentric excision due to its specific growth pattern. To achieve negative resection margins, the width of the tumor-free margins and infiltrating depth are two essential factors to be considered for complete excision for both WLE and MMS surgical techniques. Adjuvant therapy, including radiotherapy and targeted therapy, is reserved for unresectable, advanced stage, or recurrent tumors.
Keywords
dermatofibrosarcoma
wide local excision
Mohs micrographic surgery
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare superficial and uncommon low-grade soft tissue malignant sarcoma that is locally invasive and often characterized as a keloid sarcoma. It accounts for only 0.8 to 1% of all cancers and represents 1 to 1.8% of superficial soft tissue sarcomas.1 The development of DFSP was associated with chromosome 17 and 22 translocations,2 which resulted in the fusion of collagen type 1-α1 (COL1A1 at 17q22) and platelet-derived growth factor-β (PDGFB at 22q13) genes.3 Surgery options are resection with wide margins or Mohs micrographic surgery (MMS) for a limited area of body regions' excision.4 In this study, we present cases of DFSP of the head, hand, and back in Sanglah Hospital.
Case Report
Case 1
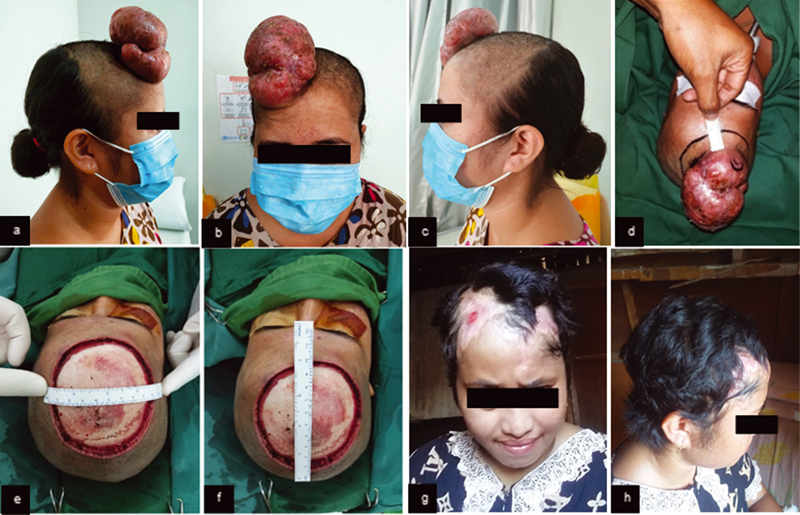
A 23-year-old female had a protuberant mass at the right forehead for 2 years before consultation and increased progressively in 2 months (Fig. 1A–C). The mass became tender for the last month. History of headache, nausea, vomiting, weight loss, similar tumor, or malignancy in the family was denied. There was an exophytic, lobulated, fixed, demarcated, solid, nontender, and coated with crusts' mass, with 6 × 10 × 5 cm in size. The mass was suggested as a soft tissue tumor by computed tomographic (CT) examination. Histopathology demonstrated well-circumscribed tumor (2 × 1 × 1 cm) as spindle-shaped subepithelial cells hyperplasia, in a “whorled” focal arrangement around collagen stroma and made a storiform structure, with monomorphic nuclei and eosinophilic cytoplasm. The patient underwent wide excision and rotational flap (Fig. 1E, F).
-
Fig. 1 (A) Preoperative mass clinical features (right lateral view). (B) Preoperative mass clinical features (anterior view). (C) Preoperative mass clinical features (left lateral view). (D) Incision marker of 2 cm distance. (E–F) Clinical features of intraoperative wide excision. (G–H) Six months after wide excision and rotational flap.
Case 2
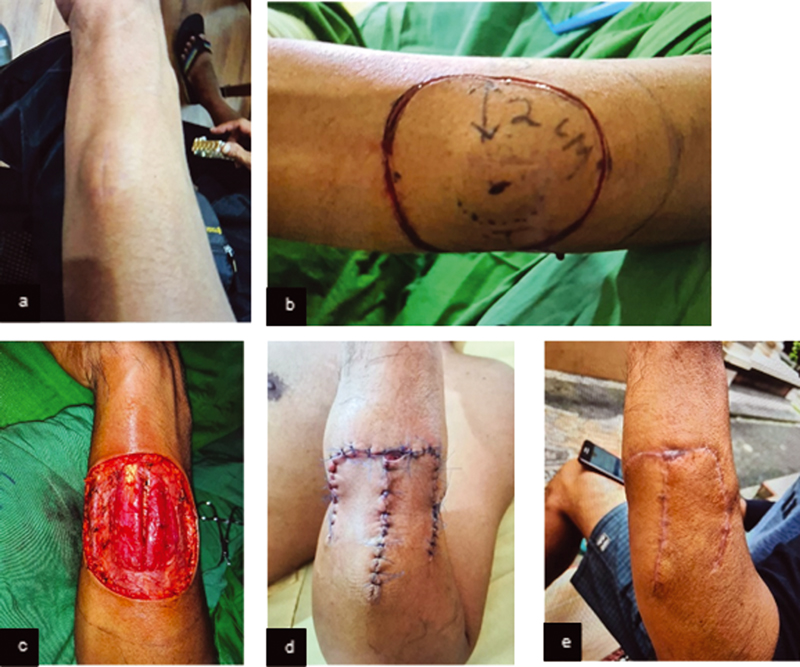
A middle-age male had a protuberant ovoid, firm, fixed, painless mass at the left proximal forearm in 6 cm size without localized heat or redness (Fig. 2A). The lesion was a small pimple 11 months before admission. No history of pain, scar, trauma around the lesion, limited range of movement, fever, weight loss, or malignancy. No palpable cervical or axillary lymph nodes were found. CT examination revealed well-defined, superficial masses with subcutaneous fat tissue infiltration. Histopathology demonstrated monomorphic fusiform cell neoplasm, in a “swirled” focal arrangement, infiltrative of dermal tissue, compatible with DFSP. The patient underwent surgical resection (Fig. 2B–D).
-
Fig. 2 (A) Preoperative mass clinical picture. (B) Incision marker of 2 cm distance. (C) Clinical features of intraoperative wide excision. (D) Postsurgical resection: day 3. (E) Three months postsurgical resection.
Case 3
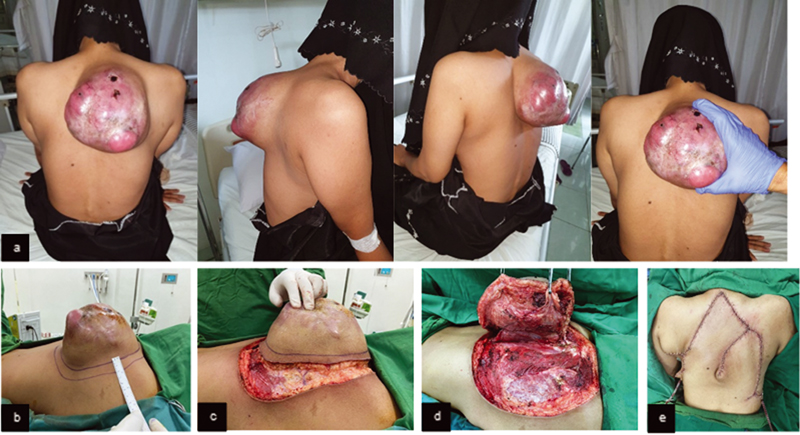
A 41-year-old female patient had a red-colored, raised, multinodular, subcutaneous mass with size 18 × 18 cm and overlying telangiectasia at the upper back. It was firm, mobile, well-demarcated with slight tenderness (Fig. 3A). The mass started as small as a marble 2 years ago, had excised but gradually grew again. X-ray showed soft tissue mass on the right thorax, passing the midline, at the level of 2nd to 8th thoracal vertebrae. CT scan demonstrated a soft tissue solid mass with necrotic components on the wall of posterior thorax, no bone destruction or erosion. Histopathological examination showed malignant spindle cell tumor consistent with the features of fibrosarcoma. The patient underwent wide excision with flap reconstruction (Fig. 3B–E).
-
Fig. 3 (A) Preoperative mass clinical picture. (B) Incision marker of 2 cm distance. (C–D) Clinical features of intraoperative wide excision. (E) Post wide excision and flap reconstruction.
Discussion
DFSP is the most frequent low-grade skin sarcoma. The annual incidence of DFSP in the United States was 4.2 per million people (1973–2002) and 4.1 per million people (2000–2010).1 The prevalence of dermatofibrosarcoma in Indonesia is not yet well documented. However, it was the most prevalent malignant soft tissue tumor in North Sumatra (2016–2017).5 Different studies reported slight male or female predominance (1:1) and arose in mid-adult age (20–50 years old).6
DFSP was characterized as slow-growing, small, firm, skin-colored, asymptomatic, painless, nonprotuberant dermal plaque in early stages (Table 1). The indurated nodule was evolved from violaceous to a red-brown irregular tumor fixed to dermis, commonly asymmetric with ill-defined margins and finger-like projections.7 The mass of all cases in this study was initially small and nontender, but gradually became a conspicuous protuberant ovoid mass.
|
Stage |
Criteria |
|---|---|
|
Stage I |
Nonprotuberant lesions including atrophic or sclerotic plaque, macula, or small nodules |
|
Stage II |
Protuberant primary tumor |
|
Stage IIA |
Superficial tumor: without invasion of the underlying fascia |
|
Stage IIB |
Deep tumor: either superficial to the fascia with infiltrating the fascia or occurred beneath the superficial fascia |
|
Stage III |
Lymph node metastasis |
|
Stage IV |
Distant metastasis to other organs |
Source: Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med 2020;9(6):1752. doi:10.3390/jcm9061752.
DFSP often develops in non-sun-exposed areas, such as trunk (42–60%), proximal extremities (23–25%), lower extremities (18%), and head and neck (10–16%).8 This tumor is locally invasive and can invade up to approximately 0.3 to 12 cm peripherally and vertically deep into internal organs, such as viscera and bones. It also initially appears like an old scar, benign cyst, or even arising from pre-existing keloid scars making it harder to establish the diagnosis since the patient will not be concerned. Given their malignant potential, DFSP rarely metastasizes. It has a high recurrence rate, especially in cases where the tumor is not completely resected deeply, and negative margins are not obtained.9 A modified staging system of DFSP based on European consensus guidelines was essential to determine the treatment.10
The standard diagnostic workup for DFSP is a histopathological examination or immunohistochemical features. Grossly, DFSP appears white to yellow, poorly circumscribed, asymmetric mass with solid and fish flesh-like texture. In some larger tumors, hemorrhagic or cystic changes may be observed.10 Histological features show spindle cell proliferation in an irregularly whorled or storiform pattern, often parallel to the skin surface, surrounding subcutaneous fat to form a honeycomb appearance. The tumor can penetrate surrounding tissue, invading subcutaneous tissue in irregular finger-like projections, identical to normal fibrous tracts. In the later phase, involvement of the fascia, underlying muscles, periosteum, bone, and the presence of sarcomatous changes may appear. Cases in the study had a fusiform cell neoplasm in a “swirled” focal arrangement. Despite the classic pattern, it required immunohistochemical staining to differentiate DFSP from other tumors. It was characterized by expression of CD34 and negative for factor XIIIa. The sensitivity of CD34 immunohistochemistry in DFSP ranges from 84 to 100%.11
Imaging diagnosis workup was done in this study. X-rays and CT scan examination are not indicated for diagnosing yet to detect some local bone destructions or pulmonary metastases that occasionally happen in patients with advanced, recurrent, or intermediate-grade tumors.10 DFSP appears as a solitary, well-defined isodense cutaneous or subcutaneous nodule with no calcification from CT examination.12
Treatment options for DFSP include surgical excision, adjuvant radiation, or targeted therapy. The primary treatment for any stage of DFSP is surgical excision. There are currently three surgical techniques to remove DFSP mass: wide local excision (WLE) with tumor-free margins, MMS, and partial or total amputation in cases where the tumor is located on the upper or lower digits. The goal of surgical excision is to achieve negative resection margins. Thus, the width of the tumor-free margins is an essential factor to be considered for complete excision for both WLE and MMS surgical techniques.13
WLE is a method of performing a three-dimensional excision including normal skin, subcutaneous tissue, and the underlying fascia within 2 to 4 cm margins from the gross tumor boundary. It is a relatively simple procedure, provides immediate wound repair, and is cost-effective. The local recurrence rate for WLE tends to decrease with surgical margins. WLE is recommended in patients with primary DSFP on the trunk or extremities within a 2 to 4 cm margin from tumor boundary to remove the mass with acceptable cosmesis and function outcomes altogether. The patient in case 1 was using MMS due to its benefit to resection with clear margins while maintaining good cosmetic outcomes. However, other cases were treated with wide excision surgery due to the noncosmetic sensitivity in a single-stage tumor.9
The drawback of WLE in which using wider margins of excision results in the unnecessary removal of healthy and potentially useful tissue led to Mohs invention of a new approach. MMS is a procedure of tumor removal by mapping, histopathologic examination of 100% of the margins, and further deeper or wider re-excision of an additional layer of surrounding tissue, if any residual tumor cells are visualized. This procedure will be repeated until all tumor margins are free of tumor cells. It is ideal for DFSP in cosmetically sensitive regions such as the face, scalp, neck, genitalia, and digits. The recurrence rates of WLE and MMS were 9.10 and 2.72%, respectively. However, MMS commonly requires specialized training for surgeons, is time-consuming, and may cost higher for patients or medical resources.14
The prognoses of patients with DFSP after surgical excision with negative or positive microscopic margins are commonly good. The 5- and 10-year recurrence-free survival rates of DFSP are 86 and 76%. In patients with positive margins after surgery, the frequencies of local recurrences ranged from 20 to 50%. Detection of metastases should be done through a thorough history and clinical examination; additional imaging examinations may also be considered. Patients should be reevaluated every 6 months for the first 5 years and yearly afterward.15
Conclusion
DFSP is rare superficial soft tissue malignant sarcoma with a slow growth, which frequently occurs in middle-aged populations. The standard diagnostic workup for DFSP is histopathological examination. Several surgical excision methods are available, including WLE, MMS, and partial or total amputation.
Conflict of Interest
None declared.
References
- Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42(01):S24-S31.
- [Google Scholar]
- Dermatofibrosarcoma protuberans: a rare entity and review of the literature. J BUON. ;19(01):34-41. http://www.ncbi.nlm.nih.gov/pubmed/24659640 . Accessed June 2, 2022
- [Google Scholar]
- A novel chromosomal translocation associated with COL1A2-PDGFB gene fusion in dermatofibrosarcoma protuberans: PDGF expression as a new diagnostic tool. JAMA Dermatol. 2015;151(12):1330-1337.
- [Google Scholar]
- Dermatofibrosarcoma protuberans on adult toes: a case report and review of the literature. Anticancer Res. 2019;39(04):2105-2111.
- [Google Scholar]
- Profil Penderita Soft Tissue Tumor Di Laboratorium Patologi Rumah Sakit Advent Medan Tahun 2016–2017. J Univ Sumatera Utara. 2020;2(18):35-38.
- [Google Scholar]
- Dermatofibrosarcoma protuberans: a systematic review of the literature. Int J Med Dev Ctries. 2020;04(11):2014-2017.
- [Google Scholar]
- Dermatofibrosarcoma protuberans in children: an update on the diagnosis and treatment. Pediatr Dermatol. 2012;29(06):707-713.
- [Google Scholar]
- Pitfall management of dermatofibrosarcoma protuberans on right leg. Int J Res Med Sci. 2019;7(09):3586.
- [CrossRef] [Google Scholar]
- Dermatofibrosarcoma protuberans: our experience of 59 cases. Oncol Lett. 2012;4(05):1047-1055.
- [Google Scholar]
- Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9(06):1752.
- [CrossRef] [Google Scholar]
- STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38(04):552-559.
- [Google Scholar]
- Imaging features of dermatofibrosarcoma protuberans with pathologic correlation and assessments for recurrence potential.
- Dermatofibrosarcoma protuberans: surgical management of a challenging mesenchymal tumor. World J Surg Oncol. 2019;17(01):90.
- [CrossRef] [Google Scholar]
- Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. J Plast Reconstr Aesthet Surg. 2014;67(10):1315-1321.
- [Google Scholar]
- Recurrent dermatofibrosarcoma protuberans: challenging a surgeon's dexterity for the ‘tricky’ margins. Ecancermedicalscience. 2018;12
- [CrossRef] [Google Scholar]







