Translate this page into:
Comparison between Vinorelbine–Carboplatin and Vinorelbine–Cisplatin in Stage III–IV EGFR Mutations-Negative NSCLC
Address for correspondence Gatot Soegiarto, PhD, Division of Clinical Immunology, Department of Internal Medicine, Faculty of Medicine, Universitas Airlangga—Dr. Soetomo General Academic Hospital, Jalan Mayjen, Prof. Dr. Moestopo No. 6–8, Airlangga, Gubeng, Surabaya, East Java 62086, Indonesia. gatot_soegiarto@fk.unair.ac.id
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction There are a substantial number of lung cancer patients with negative mutations in Indonesia. This type of cancer is deemed to be the major contributor of lung cancer patient’s death. However, reseaerch related to therapy using vinorelbine combined with platinum-based compounds is still scarce in Indonesia. The aim of this study was to compare the efficacy and tolerability between vinorelbine and carboplatin with vinorelbin and cisplatin in stage III-IV epidermal growth factor receptor (EGFR) mutations-negative non-small cell lung cancer (NSCLC).
Methods The participants were divided into two groups—group I(vinorelbine–carboplatin) and group II (vinorelbine–cisplatin). The participants were assessed based on several measurement criteria. Not only Eq-5D was performed, but the body weight and response evaluation criteria for solid tumors (RECIST) were also examined. The participants received chemotherapy for four cycles (1 cycle = 21 days).
Results The quality of life was considered stable in 60% of group I and 60% of group II (p = 0.255). In both groups, 46.67% of participants had an increased body weight, while the other 20.00% was stable (p = 1.000). In terms of RECIST evaluation after the second cycle, 80.00% of group I and 86.67% of group II were considered to have a stable disease, with 20% of group I and none of group II had partial response (p = 0.027). However, after the fourth cycle, there were no significant difference between the groups (p = 0.734).
Conclusion In EGFR mutation-negative NSCLC patients, the combination of vinorelbine and carboplatin showed comparable outcomes to vinorelbine and cisplatin chemotherapy with no significant differences.
Keywords
vinorelbine
carboplatin
cisplatin
EGFR mutation negative
NSCLC
Introduction
Lung cancer is the most common type of malignancy in the world. Based on data from the American Cancer Society in 2016, there were 223,390 new cases of lung cancer, which comprise around 14% of all cancer cases. Lung cancer is the most common cause of all cancer death, i.e. 27% in males and 26% in females. Based on reports of cancer profiles to World Health Organization (WHO), in Indonesia, lung cancer is the most common cancer occurring in males and the fifth most common cause of cancer in females after breast, cervical–uterine, colorectal, and ovarian cancers.1,2
Lung cancer is divided into non–small cell lung carcinoma (NSCLC) and small cell lung cancer (SCLC). This classification is used to determine the proper therapy. NSCLC is the most common type (70–80%) of all lung cancers and histologically consists of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, or a mixture of all three.3,4
Based on data from several hospitals in Indonesia, most lung cancer patients come and are diagnosed at an advanced stage (III–IV). Standard management options for advanced lung cancer are chemotherapy. Chemotherapy can also be used in combination with radiotherapy. The American College of Chest Physician (ACCP) guideline for epidermal growth factor receptor (EGFR)–negative NSCLC is platinum-based doublet chemotherapy, which combines platinum agents such as cisplatin or carboplatin with one of the following agents: vinorelbine, docetaxel, gemcitabine, or paclitaxel.4,5,6
In a meta-analysis of randomized clinical trials comparing cisplatin and carboplatin in NSCLC patients, it was stated that cisplatin had a better response rate than carboplatin although it was not statistically significant. Another study comparing cisplatin and gemcitabine combination and carboplatin–gemcitabine combination chemotherapy reported a significant longer survival rate of 11% in cisplatin and gemcitabine group.6,7
Several studies have reported that cisplatin has a better response rate and overall survival. However, the side effect that needs to be considered in cisplatin is nephrotoxicity.7,8 Almost all chemotherapy including vinorelbine–carboplatin and vinorelbine–cisplatin had been registered in Indonesian national drug formulary. There is currently no data on the efficacy of vinorelbine combined with platinum-based compounds in several Indonesian hospitals. Therefore, the aim of this study was to compare the efficacy of vinorelbine–carboplatin and vinorelbine–cisplatin in stage III–IV EGFR mutation–negative NSCLC.
Methods
The participants in this study were lung cancer patients based on WHO criteria.9,10,11 The inclusion criteria were male or female patients, aged 30 to 75 years, diagnosed of NSCLC based on histological pattern,12,13 EGFR mutation–negative, stage III–IV NSCLC, no chemotherapy received, measurable NSCLC (>10 mm on computed tomography [CT] scan/magnetic resonance imaging [MRI] and/or >20 mm on chest X-ray), and fulfilling the standard chemotherapy.14,15 The exclusion criterion was lung cancer due to metastasis from other organs. All of the participants received information about the study and agreed to give their written consent.
This study was an observational analytic study with a prospective design. The study was conducted from January to December, in 2017. The number of participants used in this study were 30 (group I = 15 and group II = 15). The participants were divided into two groups—group I received vinorelbine–carboplatin therapy and group II received vinorelbine–cisplatin therapy (Fig. 1). EGFR mutation–negative test was conducted by using the QIAamp deoxyribonucleic acid (DNA) mini kit (QIAamp, Valencia, California, United States) and the High Pure PCR Template Preparation (Roche Diagnostics, Indianapolis, United States). Vinorelbine was given to both groups intravenously at a dose of 25 mg/m2. One cycle of vinorelbine was given on the first day and the eighth day at the beginning of therapy. Carboplatin was given to the first group intravenously at a dose of 300 mg/m2 (AUC-5). Meanwhile, the second group was given additional therapy of cisplatin at a dose of 60 mg/m2. The present study was approved by the institutional ethics committee.
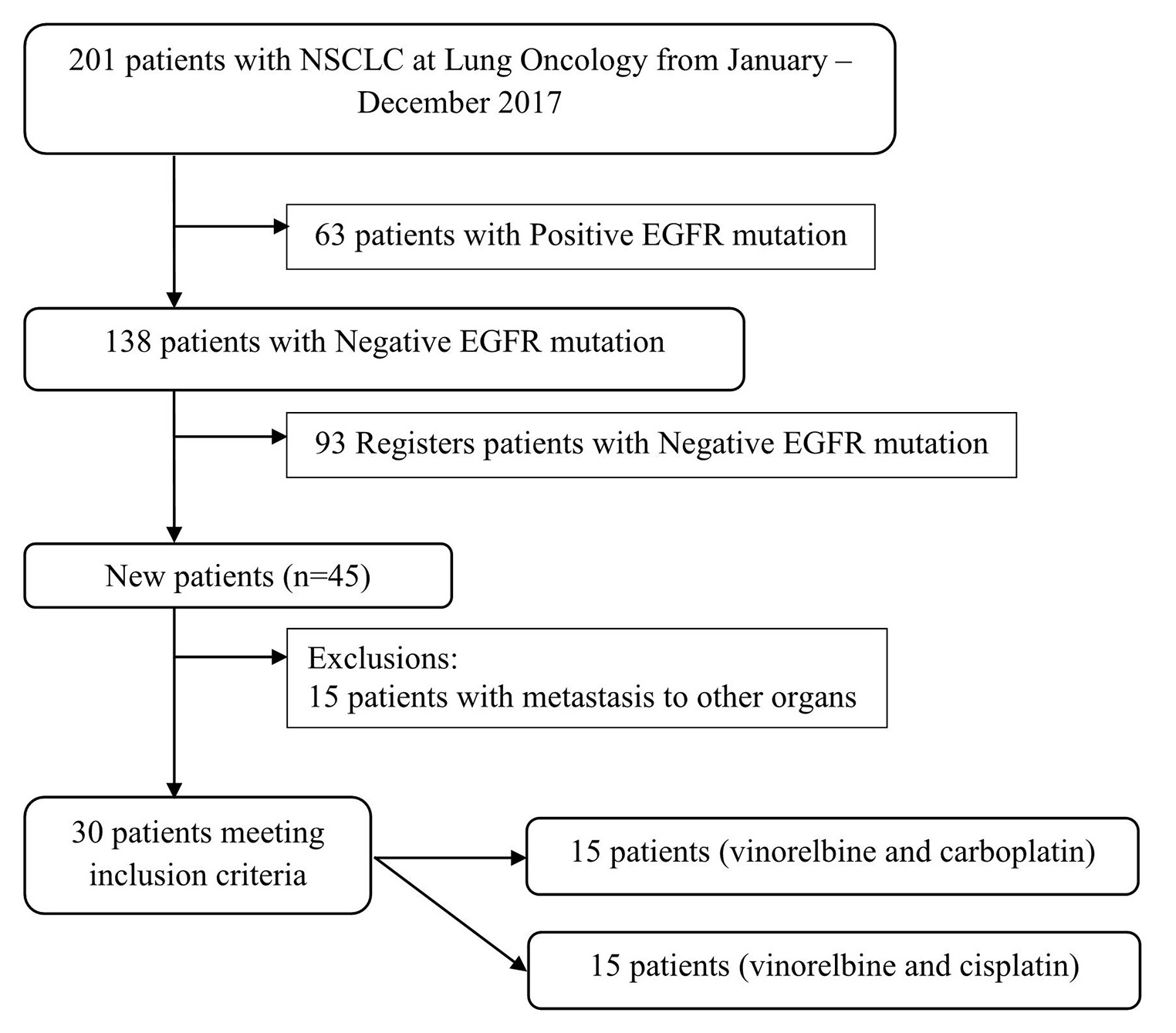
-
Fig. 1 Recruitment process on the participant.
The quality of life of participants before and after administration of vinorelbine–carboplastin (group 1) and vinorelbine–cisplatin (group II) were assessed using EuroQol Five-Dimensions (Eq-5D) questionnaire, whereas the body weight and the evaluation of target and nontarget lesion was based on response evaluation criteria for solid tumors (RECIST). The Eq-5D questionnaire was adopted from Khan et al16,17 which was declared valid and reliable (Cronbach’s α > 0.7).18 The Eq-5D questionnaire score was categorized into 3 groups, i.e. decreased, stable, or increased score; which respectively signify an improved, stable, or deteriorated quality of life. In this study, Eq-5D in the Indonesian language was used. Assessment of body weight involved the use of a weight scale that had been calibrated before. Body weight was categorized into three groups, namely, weight gain, stable weight, and weight loss. Assessment of RECIST involved the use of thoracic CT scan. For CT scans Siemens 128-slice scanners (Cement, Erlangen, Germany) were used. Thoracic CT images were obtained from the pulmonary apex to the base, as long as inspiration was suspended, in one breath with intravenous contrast. Assessment of thoracic CT scan was divided into 4 groups, namely, progressive disease, stable disease, partial response, and complete response. Progressive disease is an increase in target lesion size of ≥20% from the previous size. Partial response is a reduction in target lesion size of ≥30% compared with the target lesion diameter at baseline. Stable disease, which is a stagnant condition or a measure of target lesion, does not meet the criteria for progressive disease and partial response. Complete response is the evaluation of the target adenocarcinoma or lychee missing 100% and the lymph nodes shrink to <10 mm.19
The procedure of research included dividing participants into two groups (groups I and II) and giving chemotherapy (vinorelbine and carboplastin or vinorelbine and cisplatin). Previously, the participants were examined by Eq-5D questionnaire, weight body, and RECIST evaluation. Chemotherapy was given for 4 cycles where 1 cycle of chemotherapy was conducted every 21 days. Group I was given vinorelbine and carboplatin therapy on the first day intravenously, whereas group II was given vinorelbine and cisplatin on the first day intravenously. Furthermore, groups I and II were given additional vinorelbine therapy on the eighth day. Chemotherapy evaluation was performed after the second and fourth cycles. The participants also were also evaluated on the side effects of chemotherapy in fourth cycles.
Univariate data were analyzed based on data types (numeric or categorical). The collected data initially were assessed by using Shapiro–Wilk normality test. The data analysis for quality of life (Eq-5D) and body weight participant used independent t-test or Mann–Whitney U test. For comparison of groups I and II with RECIST evaluation Mann Whitney U test was used. The statistical analysis in side drug used chi-square test or Fisher’s exact test. The odds ratio (OR) and 95% confidence interval (CI) were used to estimate risk; p < 0.05 was accepted as statistically significant. The statistical analysis was performed by applying IBM SPSS Statistics software version 23.0 (IBM Corp., Armonk, New York, United States).
Results
Characteristics of Participants
The majority of participants were males in group I (93.33%) and group II (93.33%). The average age of participants in group I was 55.00 ± 12.92 years and in group II it was 57.33 ± 8.96 years. Participant age range was between 21 and 76 years. Most of the participants were smokers in group I, with a percentage of 80.00%, and the percentage of smokers in group II was 93.33%. The average of RECIST evaluation were 86.67 ± 4.88 (group I) and 84.00 ± 5.07 (group II). All histopathological diagnosis of adenocarcinoma was established based on fine needle aspiration biopsy (FNAB) thoracic. Most of participants in group I (80%) and in group II (66.67%) had adenocarcinoma stage IV (Table 1).
|
Variable |
Group I (n = 15) |
Group II (n = 15) |
|---|---|---|
|
Gender |
||
|
Male |
14 (93.33) |
14 (93.33) |
|
Female |
1 (6.67) |
1 (6.67) |
|
Smoking status |
||
|
Smoker |
12 (80.00) |
14 (93.33) |
|
Nonsmoker |
3 (20.00) |
1 (6.67) |
|
Stage |
||
|
IIIB |
3 (20.00) |
5 (33.33) |
|
IV |
12 (80.00) |
10 (66.67) |
|
Physical statusa |
||
|
80 |
5 (33.33) |
9 (60.00) |
|
90 |
10 (66.67) |
6 (40.00) |
Abbreviations: Group I, vinorelbine–carboplatin therapy usage; group II, vinorelbine–cisplatin therapy usage.
aMean of physical status was 86.67 ± 4.88 (group I) and 84.00 ± 5.07 (group II).
Quality of Life (Eq-5D)
Quality of life of the participants based on the Eq. 5D questionnaire was assessed 3 times (initial assessment and postchemotherapy second and fourth cycles). The quality of life of NSCLC patients did not have a significant difference pre- and postchemotherapy (Table 2). Most participants (groups I and II) were stable at 60.00% and there were no significant differences between the quality of life of NSCLC group I and group II patients (p = 0.255; Table 3).
|
Variable |
Mean ± SD/median (min–max) |
p-Valuea |
|
|---|---|---|---|
|
Group I |
Group II |
||
|
Eq. 5D |
|||
|
Start |
5.00 (5.00 – 7.00) |
6.00 (5.00 – 8.00) |
0.059 |
|
Second cycles |
5.60 ± 0.73 |
6.13 ± 1.18 |
0.150 |
|
Fourth cycles |
5.00 (5.00 – 10.00) |
6.00 (5.00 – 8.00) |
0.940 |
|
Body weight |
|||
|
Start |
49.40 ± 8.45 |
50.87 ± 9.87 |
0.665 |
|
Second cycles |
49.40 ± 8.55 |
50.67 ± 10.16 |
0.150 |
|
Fourth cycles |
50.07 ± 8.23 |
51.62 ± 10.98 |
0.940 |
Abbreviations: Group I, vinorelbine–carboplatin therapy usage; group II, vinorelbine–cisplatin therapy usage; NSCLC, non–small cell lung carcinoma.
aSignificant p < 0.05.
|
Variable |
n (%) |
p-Valuea |
|
|---|---|---|---|
|
Group I |
Group II |
||
|
Eq. 5D |
0.255 |
||
|
Increase |
1 (6.67) |
3 (20.00) |
|
|
Stable |
9 (60.00) |
9 (60.00) |
|
|
Decrease |
5 (33.33) |
3 (20.00) |
|
|
Body weight |
1.000 |
||
|
Enhance |
7 (46.67) |
7 (46.67) |
|
|
Stable |
3 (20.00) |
3 (20.00) |
|
|
Decline |
5 (33.00) |
5 (33.00) |
|
Abbreviations: Group I, received vinorelbine-carboplatin therapy; Group II, received vinorelbine-cisplatine therapy; NSCLC, non-small cell lung cancer. *Significant p < 0.05
.
Body Weight
Body weight of the participants was also assessed 3 times. In the first assessment, the average of body weight were 49.40 ± 8.45 (group I) and 50.87 ± 9.87 (group II; p = 0.665). In post chemotherapy second cycle, the average of body weight were 49.40 ± 8.55 (group I) and 50.67 ± 10.16 (group II; p = 0.150). In post chemotherapy fourth cycles, the average of body weight were 50.07 ± 8.23 (group I) and 51.62 ± 10.98 (group II; p = 0.940; Table 2). Body weight of the participants had the same distribution between groups I and II after conducting chemotherapy fourth cycles. It was revealed that most participants (groups I and II) experienced weight gain (46.67%; p = 1.000; Table 3).
RECIST
The participants underwent CT scan after the second and the fourth cycle of chemotherapy and compared to RECIST evaluation at baseline. In postchemotherapy second cycle, most of the participants (80% of group I and 86.67% of group II) were at stable disease. More subjects in group I experienced partial response than in group II (20.00% vs. 0.00%, p=0.027). In postchemotherapy fourth cycles, most participants in group I had progressive disease category of 40.00% and most of group II had physical condition in the stable disease category of 66.67%. There were no significant differences between groups I and II (p = 0.734; Table 4).
|
Physical condition |
n (%) |
p-Value |
|
|---|---|---|---|
|
Group I |
Group II |
||
|
Second cycles |
0.027* |
||
|
Partial response |
3 (20.00) |
0 (00.00) |
|
|
Stable disease |
12 (80.00) |
13 (86.67) |
|
|
Progressive disease |
0 (00.00) |
2 (13.33) |
|
|
Fourth cycles |
0.734 |
||
|
Partial response |
4 (26.67) |
2 (13.33) |
|
|
Stable disease |
5 (33.33) |
10 (66.67) |
|
|
Progressive disease |
6 (40.00) |
3 (20.00) |
|
Abbreviations: Group I, received vinorelbine-carboplatin therapy; Group II, received vinorelbine-cisplatine therapy; NSCLC, non-small cell lung cancer; RECIST, response evaluation criteria for solid tumors.
*Significant p < 0.05.
Adverse Effects of the Drug
Most of the participants experienced side effect of the drug when they were given chemotherapy of 73.33% (group I) and 80.00% (group II). There were no significant differences in the incidence of chemotherapy side effects in both groups (p = 1.000; Table 5). Most participants in group I had side effects in the form of nausea and vomiting, and most participants in group II also had side effect in the form of nausea and vomiting. This was followed by anemia in 33.33% (group I) and 46.67% (group II; Fig. 1).
|
Drug side effect |
n (%) |
p-Value |
|
|---|---|---|---|
|
Group I |
Group II |
||
|
Positive |
11 (73.33) |
12 (80.00) |
1.000 |
|
Negative |
4 (26.67) |
3 (20.00) |
|
Abbreviations: Group I, received vinorelbine-carboplatin therapy; Group II, received vinorelbine-cisplatine therapy.
*Significant p < 0.05.
Discussion
This study showed that, in general, there were no significant differences between vinorelbine–carboplatin and vinorelbine–cisplatin in advanced (stage IIIB–IV) pulmonary adenocarcinoma with negative EGFR mutation. Comparing the quality of life, body weight, and RECIST evaluation, we found comparable results. The patient’s quality of life as subjective response is also a very important measure. The RECIST evaluation is not always followed by a quality of life. In our study, we found that the enhanced RECIST was in accord with the quality of life, where most of the patients were at stable disease.20,21
Changes in complaints or clinical symptoms can be caused by a variety of factors such as the tumor mass, pleural effusion, and paraneoplastic syndromes. All of those measures are subjective and patients often do not consider it as a response to therapy. In advanced stage of lung cancer with WHO performance status score of 2, the most needed result of therapy was an improvement of symptoms (quality of life).22
Our study used body weight changes as a measure of body weight. Body weight changes were influenced by nutritional factors which are affected by several factors such as patient’s appetite, nutritional intake, and resting energy expenditure. Patient’s appetite on the other hand is affected by various patients’ clinical conditions such as the presence of pleural effusion, the general condition, and chemotherapeutic response. Malnutrition in cancer patients is associated with weight loss due to an increase in resting energy expenditure caused by systemic inflammatory response syndrome (SIRS). Research conducted by Arrieta et al, which assessed the relationship of nutritional status and serum albumin levels with the toxicity of cisplatin and paclitaxel combination chemotherapy, found the prevalence of malnutrition in lung cancer.
Several other studies using the same regimen of chemotherapy also reported a change in body weight in 40-80% of their study subject.23 Platinum-based regimens gave similar results with the current study in terms of RECIST evaluation. The RECIST evaluation was dominated by stable disease. A study by the Southwest Oncology Group (SWOG) comparing single cisplatin with cisplatin and vinorelbine combinations showed that the combination of cisplatin–vinorelbine was better with a response rate of 26% versus 12%.24 A follow-up study by SWOG comparing the combination of paclitaxel–carboplatin to vinorelbine–cisplatin found no significant difference in the efficacy and response rate of both combination regimens. However, the cost of the paclitaxel–carboplatin combination regimen was higher.24,25
Study by He et al found that patients who received cisplatin + etoposide combination chemotherapy yielded partial response in 20.3%, stable disease in 35.9%, and progressive disease in 31.3% of the participants. No complete response was achieved in that study.26 Research by Wahl et al attained a complete response of 4%, partial response of 13%, stable disease of 33%, and progression disease of 24%.27 Multicenter clinical trial by Ozkaya et al compared cisplatin–vinorelbine chemotherapy to cisplatin–gemcitabine and showed no difference in terms of RECIST evaluation, clinical response rates, time to disease progression, and overall survival.28
Several studies to compare the efficacy of various platinum-based regimens have been conducted in some developed countries. The platinum-based first-line chemotherapy response remains superior even though the toxicity was more common. The survival rates did not show any statistical differences. Another study that compared the benefits of single chemotherapeutic agent (vinorelbine or gemcitabine) with combination of both drugs in elderly lung cancer patients (older than 70 years) found that the drug efficacy was similar but the toxicity was higher in the combination regimen.25,29
The results of our study were in accordance with several other studies which generally showed that there was no superiority among various combination regimens.30 Thus, in source-limited countries, only the cost of therapy and patient preference can be used as considerations in deciding which regimen that will be given to the patient.
The hematological toxicity of chemotherapeutic agents is due to their myelosuppressive effects. A chemotherapeutic agent not only kills cancer cells but it also affects normal cells that are actively dividing such as hematopoietic cells in the bone marrow. Progenitor cells that give rise to granulocytes, erythrocytes, and platelets in the peripheral blood circulation will be destroyed. In several studies, it has been asserted that carboplatin has more dominant hematological toxicity, whereas cisplatin has greater gastrointestinal toxicity than carboplatin. Cisplatin toxicity was more common at high doses (100–120 mg/m2) and may manifest as renal toxicity, auditory toxicity, or neurotoxicity.20,31 In the present study, either virrelbine–carboplatin and vinorelbine–cisplatin combination caused comparable grade 1 and 2 toxicities which are generally tolerated by the patients.
Conclusion
Chemotherapeutic combination remains the recommended treatment for advanced pulmonary adenocarcinoma with negative EGFR mutation. This study proved that vinorelbine-carboplatin or vinorelbine-cisplatin yielded comparable results in terms of quality of life, body weight change, and physical conditions (performance status), although vinorelbine-cisplatin combination showed slightly less patients with progressive disease. Vinorelbine and carboplatin combination resulted in higher proportion of hematological toxicities, while vinoprelbine and cisplatin combination resulted in more of nonhematological toxicities, but all of them were generally tolerable.
Note
All procedures performed in studies involving human participants were in accordance with the ethical standards according to Helsinki declaration at the ethical committee of Dr. Soetomo General Academic Hospital, Surabaya, Indonesia, with Certificate number 193/Panke. KKE/III/2017.
Acknowledgments
The authors extend gratitude to the Director of the Dr. Soetomo General Academic Hospital, Surabaya, Indonesia for his full support in the conduct of this study. They would also like to thank Fis Citra Ariyanto for editing the manuscript.
Conflict of Interest
None declared.
Note
This article has been published internally by Universitas Airlangga (http://repository.unair.ac.id/74575/).
References
- 2012. Principles and Practice of Lung Cancer: The Official Reference Text of the International Association for the Study of Lung Cancer (IASLC). Philadelphia, PA: Lippincott Williams and Wilkins
- Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis.. 2010;2(01):48-51. Mar;
- [Google Scholar]
- International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(08):706-714.
- [Google Scholar]
- Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(03):266S-276S. (Suppl)
- [Google Scholar]
- Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(03):277S-289S. (Suppl)
- [Google Scholar]
- Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18(02):317-323.
- [Google Scholar]
- Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(03):234S-242S. (Suppl)
- [Google Scholar]
- Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician. 2015;91(04):250-256.
- [Google Scholar]
- Comparison of vascular endothelial growth factor-A (VEGF-A) level in pleural fluid of patients with malignant and nonmalignant pleural effusion. Indian J Surg. 2021;83(03):132-138.
- [Google Scholar]
- Sahrun. Comparison of Detection of Epidermal Growth Factor Receptor (EFGR) Gene Mutation in Peripheral Blood Plasma (Liquid Biopsy) with Cytological Specimens in Lung Adenocarcinoma Patients. Indian J Surg Oncol 2020 (e-pub ahead of print). doi: https://doi.org/
- [CrossRef] [Google Scholar]
- T790M mutations identified by circulating tumor DNA test in lung adenocarcinoma patients who progressed on first-line epidermal growth factor receptor-tyrosine kinase inhibitors. Lung India. 2020;37(01):13-18.
- [Google Scholar]
- Treatment of non-small cell lung cancer (NSCLC) J Thorac Dis. 2013;5(04):S389-S396. (Suppl 4)
- [Google Scholar]
- First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5(02):260-274.
- [Google Scholar]
- Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14(01):60-75.
- [Google Scholar]
- A non-linear beta-binomial regression model for mapping EORTC QLQ- C30 to the EQ-5D-3L in lung cancer patients: a comparison with existing approaches. Health Qual Life Outcomes. 2014;12(01):163-179.
- [Google Scholar]
- Validation of the EuroQol Five-dimensions—Three-Level Quality of Life instrument in a classical Indian language (Odia) and its use to assess quality of life and health status of cancer patients in eastern India. Indian J Palliat Care. 2015;21(03):282-288.
- [Google Scholar]
- Response Evaluation Criteria in Solid Tumors (RECIST) criteria are superior to European Association for Study of the Liver (EASL) criteria at 1 month follow-up for predicting long-term survival in patients treated with transarterial chemoembolization before liver transplantation for hepatocellular cancer. J Vasc Interv Radiol. 2013;24(06):805-812.
- [Google Scholar]
- Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: a cooperative multinational trial. Ann Oncol. 2002;13(10):1539-1549.
- [Google Scholar]
- Estrogen and progesterone receptors in non small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2002;8(02):69-73.
- [Google Scholar]
- 2016. Muray and Nadels Textbook of Respiratory Medicine. 6th Edition. Elsevier Saunders: Philadelphia, PA
- Respons dan toleransi pasien adenokarsinoma paru stage III dan IV untuk pemberian kemoterapi dengan rejimen paclitaxel (Paxus®) plus carboplatin. J Respir Indo. 2010;30(02):105-111.
- [Google Scholar]
- P2–215: outcome of advanced stage non-small-cell lung cancer patients who have received combination carboplatin plus etoposide chemotherapy with radiotherapy in Persahabatan Hospital, Jakarta, Indonesia. J Thorac Oncol. 2007;2(08):S657.
- [Google Scholar]
- Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005;16(07):1069-1075.
- [Google Scholar]
- Initial partial response and stable disease according to RECIST indicate similar survival for chemotherapeutical patients with advanced non-small cell lung cancer. BMC Cancer. 2010;10:681-692.
- [Google Scholar]
- From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(01):122S-150S. (Suppl 1)
- [Google Scholar]
- Comparison of vinorelbine-cisplatin with gemcitabine-cisplatin in patients with advanced non-small cell lung cancer. Clin Med Circ Respirat Pulm Med. 2008;2:27-34.
- [Google Scholar]
- Usefulness of serum carcinoembryonic antigen (CEA) in evaluating response to chemotherapy in patients with advanced non small-cell lung cancer: a prospective cohort study. BMC Cancer. 2013;13:254-261.
- [Google Scholar]
- Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(02):92-98.
- [Google Scholar]
- Chemotherapy related toxicity in locally advanced non-small cell lung cancer. J Cancer Res Ther. 2006;2(01):14-16.
- [Google Scholar]








