Translate this page into:
Clinical Profile and Outcomes of Treatment in Gastric Cancer in Young Patients in India
Address for correspondence Venkata Pradeep Babu, MD, DNB, Department of Medical Oncology, Assam Cancer Care Foundation, ACCF Onco Care, Near Tumuki Stadium, Tezpur, Assam, 784153, India. pradeepbabu.koyyala@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction Gastric cancer poses an enormous burden across the globe and India in terms of cancer-related mortality. There is paucity of epidemiological and survival data among young gastric cancer patients in India. In this study, we retrospectively analyzed the general characteristics, clinical profile, and survival data of gastric cancer in young patients < 30 years at tertiary care institution at New Delhi, India.
Materials and Methods Young gastric cancer patients (≤30 years) who were registered over a period of 7 years (2010–2017) were analyzed at a tertiary care center. Total of 2,735 patients of gastric cancers were registered out of which 70 cases were younger than 30 years, of which 63 patients were available for final analysis and data was missing for the remaining 7 cases. All patients underwent standard diagnostic and staging investigation and were staged as per American Joint Committee on Cancer 7 staging system. Lymph node ratio was calculated as number of positive nodes by the number of lymph nodes removed and were categorized as ≤0.6 and >0.6. Minimum follow-up of 1 year was required for inclusion in the study. Twelve patients were lost to follow-up and were not included for survival analysis.
Results Younger patients (≤30 years) with gastric cancer were 2.5% of total gastric cancer patients. Mean age was 24.9 years with males being involved twice as commonly as females (2.15:1). Positive family history was present in 14.2% patients and smoking was present in 57.1% patients. Metastatic disease at presentation was present in 69.8% patients, while only 6.4% patients presented with stage I/II disease. Fourteen patients underwent surgery, out of which six patients underwent partial gastrectomy and remaining eight underwent total gastrectomy with D2 nodal dissection. Median overall survival was 10.8 months (8.8–12.8) and 2-year overall survival was 15.1%.
Conclusion Incidence of stomach cancer in young patients is more than expected and more than global average in India. Most of these young patients are presenting in advanced stage and survival is poor compared with typical aged patients
Keywords
cancer in young patients
gastric cancer
stomach cancer
Introduction
Gastric cancer is a deadly disease. It currently ranks second only to lung cancer as a leading cause of death with estimated 723,000 deaths annually worldwide.1 The incidence of gastric cancer is varied among various geographic regions; it is as high as 80 to 82 per 100,000 in some cities in Japan to as low as <10 × 100,000 in Kuwait, United States, and Mexico.2 The estimated number of new cases of stomach cancer in India in 2016 was 75,000, while the total prevalent cases were 1,12,000. From 1990 to 2016, the age-standardized incidence rate of stomach cancer has decreased.3 The age-adjusted incidence rate of stomach cancer in males varies widely among various registries in India, highest being 11.1 per 100,000 in Chennai compared with 1.6 per 100,000 in Bhopal. Stomach cancer is the only subtype among all other cancer for which the estimated incidence rate is decreasing across all the states in India.4
The 5-year survival rate for stomach cancer approximately varies from 90% for stage IA to 4% for stage IV disease.5 Survival rates are higher in countries that have screening programs that lead to early detection and where distal cancer (which has a better prognosis) predominates. The mortality figures from Indian registries suffer with problem of under-reporting because of problems in registration of death and in reporting of cause of death. The 5-year relative survival for stomach cancer in India from 1992 to 1994 was observed to be 6%.4
The substantial decrease in the age-standardized incidence rate of stomach cancer across the country might be due to lifestyle changes such as reduced consumption of salt-preserved foods, better availability of refrigeration, increasing fruit consumption, and decrease in smoking prevalence.6 The proportion of young patients with gastric cancer has varied from 6 to 8% to 2 to 6% depending on the cutoff taken as 41 years or 36 years for defining the young population. The young patients have the high frequency of advanced stage lesions and undifferentiated tumors at presentation as compared with older adults in nearly all the studies; this has often been attributed to the delay in diagnosis. Younger patients tend to have more rapidly growing and biologically aggressive tumors. Younger patients are less likely to present as gastroesophageal junction growth as compared with antral growth.7
There is paucity of epidemiological and survival data among young gastric cancer patients in India. In this study, we retrospectively analyzed the general characteristics, clinical profile, and survival data of gastric cancer in young patients < 30 years at tertiary care institution at New Delhi, India.
Materials and Methods
Young gastric cancer patients (≤30 years) who were registered over a period of 7 years (2010–2017) were analyzed at a tertiary care center. Total of 2735 patients of gastric cancers were registered out of which 70 cases were younger than 30 years, of which 63 patients were available for final analysis and data was missing for the rest 7 cases. All patients underwent standard diagnostic and staging investigation and were staged as per American Joint Committee on Cancer 7 staging system. Lymph node ratio (LNR) was calculated as number of positive nodes by the number of lymph nodes removed and were categorized as ≤0.6 and >0.6. Minimum follow-up of 1 year was required for inclusion in the study. Twelve patients were lost to follow-up and were not included for survival analysis.
Statistics
Descriptive summaries were presented in mean and standard deviation for continuous variables and frequencies with percentages for categorical variables and the results were tabulated. Primary outcome was overall survival (OS) and was calculated from date of diagnosis to the date of death and censored at last follow-up. Disease-free survival (DFS) was calculated for nonmetastatic disease and progression-free survival (PFS) was calculated for metastatic disease. Survival analysis is done by Kaplan–Meier method and is graphically represented with comparison between two factors done by log-rank test. Factors affecting OS were analyzed via Cox regression analysis and univariate and multivariate studies were accordingly tabulated (IBM SPSS Statistics for Windows, Version 25.0. IBM Corp, Armonk, New York, United States). A p-value of <0.05 was considered significant.
Results
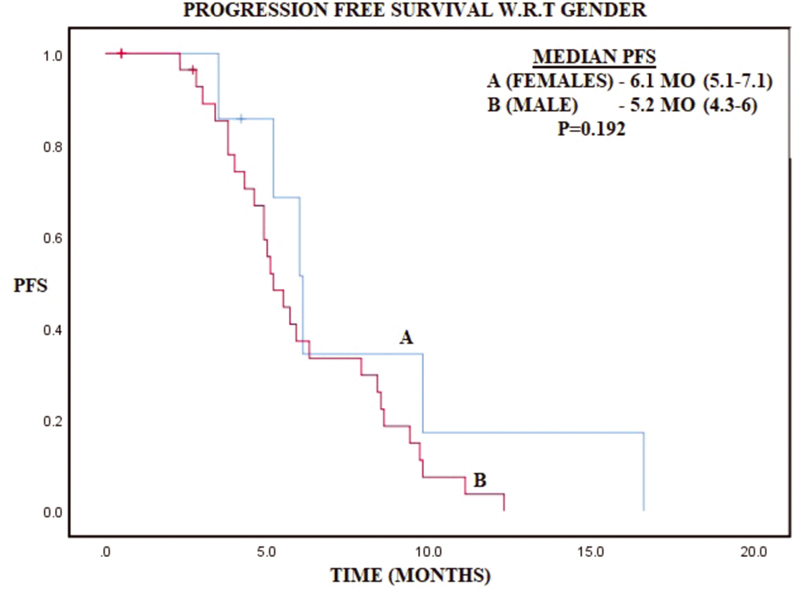
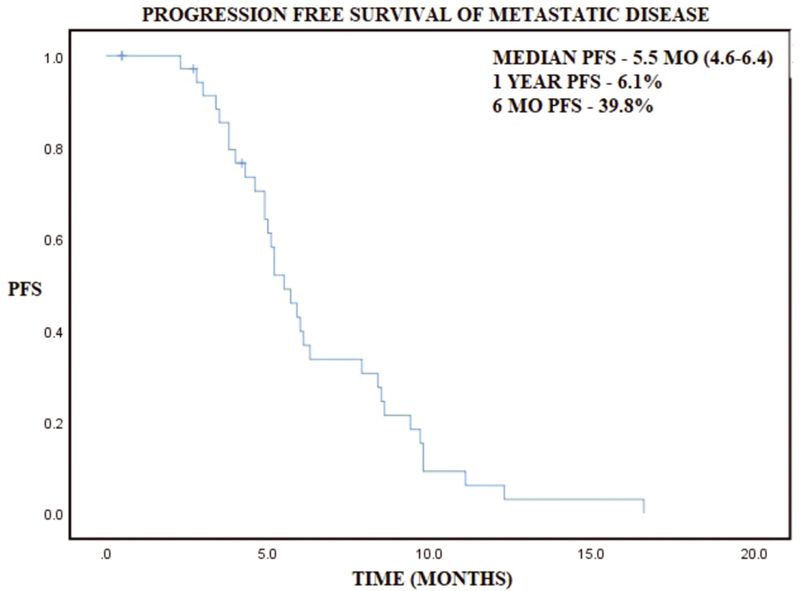
Younger patients (≤30 years) with gastric cancer were 2.5% of total gastric cancer patients. Mean age was 24.9 years with males being involved twice as commonly as females (2.15:1). Positive family history was present in 14.2% patients and smoking was present in 57.1% patients. Demographic and histopathologic profile has been represented in Table 1. Common histologies were poorly differentiated carcinoma (41.3%) and signet ring cell carcinoma (33.3%), while well and moderately differentiated histologies constituted 7.9% and 17.5%, respectively. Metastatic disease at presentation was present in 69.8% patients, while only 6.4% patients presented with stage I/II disease. Out of 19 nonmetastatic patients, 5 patients were lost to follow-up and rest 14 patients underwent surgery, out of which 6 patients underwent partial gastrectomy and rest 8 underwent total gastrectomy with D2 nodal dissection in all patients. Minimum number of nodes removed were 16 and maximum were 53, while maximum number of positive nodes were 26 and LNR of ≤0.6 and >0.6 was 7 patients each. Median OS was 10.8 months (8.8–12.8) and 2-year OS was 15.1% in patients with metastatic disease median PFS of 5.5 months (4.6–6.4) and median OS of 9.2 months (8.1–10.3) (Table 2). In patients with nonmetastatic disease, 2-year DFS and OS is 18 and 29.4%, while median DFS and OS were 10.5 and 21.6 months, respectively (Table 2). On cox regression analysis LNR >0.6 had trend toward worse survival with hazard ratio (HR) of 2.83 (0.7–11.5, p = 0.145) and similarly age > 20 had a trend toward worse survival as compared with age ≤20 with HR of 2.37 (0.83–6.73, p = 0.104) (Table 3). Kaplan–Meier graphs have been represented below for OS with respect to gender, stage, DFS in nonmetastatic disease and with respect to LNR, PFS in metastatic disease (Figs. 123456).
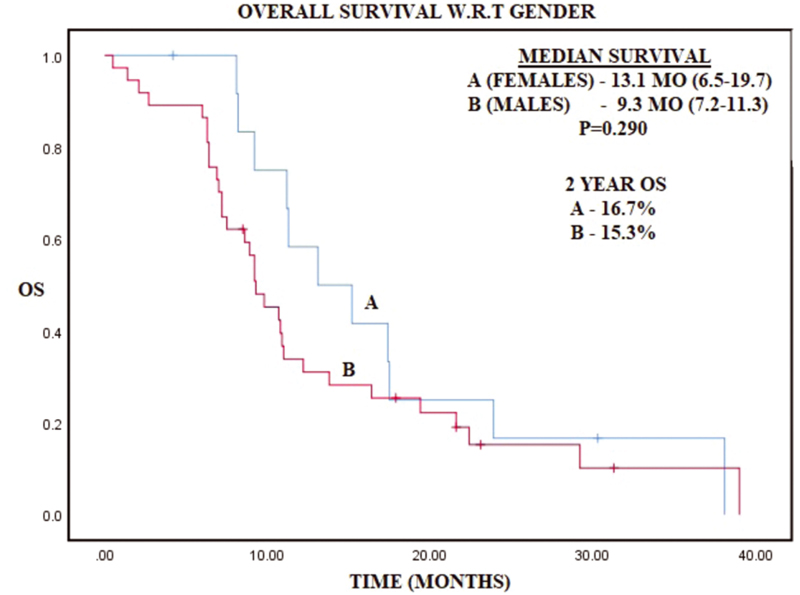
-
Fig. 1 Kaplan–Meier curve representing overall survival (OS) with respect to gender.
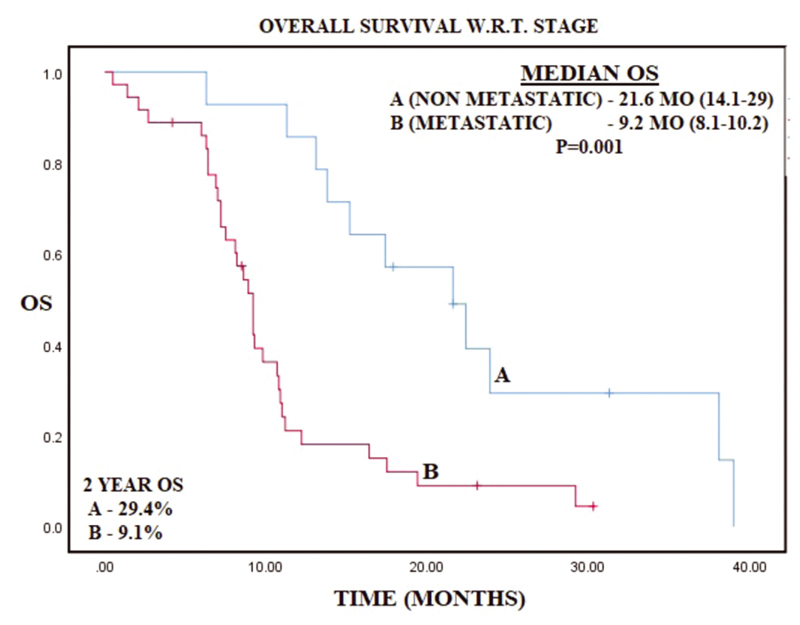
-
Fig. 2 Kaplan–Meier curve representing overall survival (OS) with respect to stage of gastric cancer.
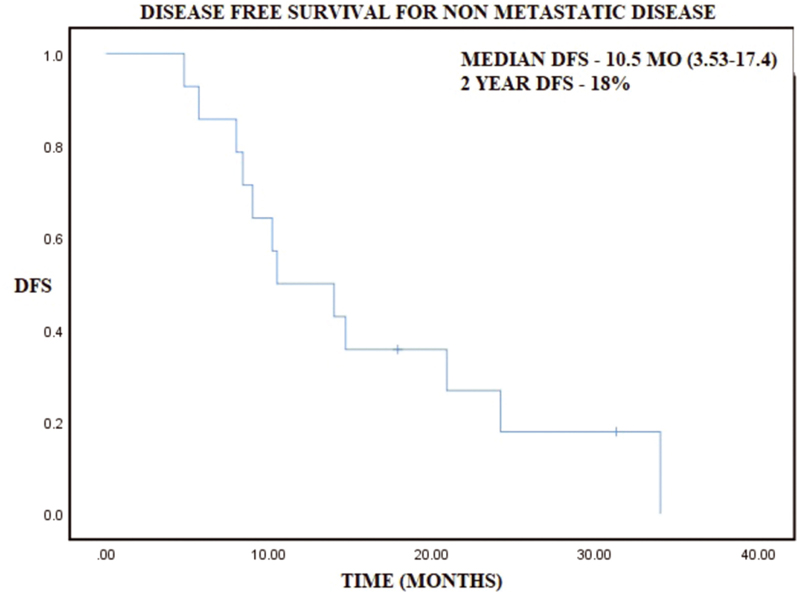
-
Fig. 3 Kaplan–Meier curve representing disease-free survival (DFS) in nonmetastatic disease.
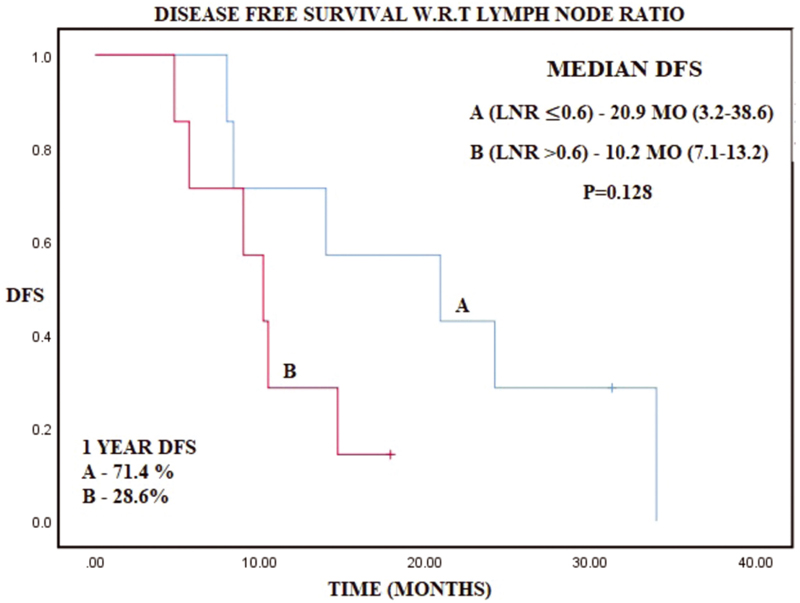
-
Fig. 4 Kaplan–Meier curve representing disease-free survival (DFS) with respect to lymph node ratio (LNR).
-
Fig. 5 Kaplan–Meier curve representing progression-free survival (PFS) in metastatic disease.
-
Fig. 6 Kaplan–Meier curve representing disease-free survival (DFS) with respect to gender.
|
Age |
24.9 ± 3.3 (17–30) |
|
Gender (M:F) |
2.15:1 (68.3/31.7%) |
|
Histology |
|
|
Well differentiated |
5/63 (7.9%) |
|
Moderately differentiated |
11/63 (17.5%) |
|
Poorly differentiated |
26/63 (41.3%) |
|
Signet ring cell |
21/63 (33.3%) |
|
Smoking |
57.1% (36/63) |
|
Positive family history |
7/49 (14.2%) |
|
Stage |
|
|
Nonmetastatic |
19/63 (30.2%) |
|
Stage I |
1/63 (1.6%) |
|
Stage II |
3/63 (4.8%) |
|
Stage III |
15/63 (23.8%) |
|
Metastatic |
|
|
Stage IV |
44/63 (69.8%) |
|
Site of metastasis |
|
|
Ascites/omentum |
26/44 (59.1%) |
|
Liver |
12/44 (27.2%) |
|
Ovary |
6/44 (13.6%) |
|
Lung |
4/44 (9.1%) |
|
Distant lymph node |
4/44 (9.1%) |
|
Adrenal |
1/44 (2.2%) |
|
Skin |
1/44 (2.2%) |
|
Median follow-up time |
31.3 MO ± 4.4 (22.5–40) |
|
Overall patients |
63 |
|
Patient evaluated for survival |
51/63 |
|
Overall survival (OS) |
|
|
2-year survival |
15.1% |
|
Median survival |
10.8 months (8.8–12.8) |
|
Metastatic disease |
|
|
Number of patients with metastatic disease |
44/63 (69.8%) |
|
Progression-free survival (PFS) |
|
|
12-month PFS |
6.1% |
|
Median PFS |
5.5 months (4.6–6.4) |
|
OS |
|
|
12-month OS |
21.2% |
|
Median OS |
9.2 months (8.1–10.3) |
|
Nonmetastatic disease |
|
|
Number of patients with nonmetastatic disease |
19/63 (30.2%) |
|
Disease-free survival (DFS) |
|
|
2-year DFS |
18% |
|
Median DFS |
10.5 months (3.53–17.4) |
|
OS |
|
|
2-year OS |
29.4% |
|
Median OS |
21.6 months (14.1–29) |
|
OS |
Univariate analysis |
|||
|---|---|---|---|---|
|
HR |
95% CI |
p-Value |
||
|
Age |
||||
|
≤20 |
1 |
|||
|
> 20 |
2.37 |
(0.83–6.73) |
0.104 |
|
|
Gender |
||||
|
Female |
1 |
|||
|
Male |
1.45 |
(0.72–2.9) |
0.295 |
|
|
Histology |
||||
|
Nonsignet ring cell |
1 |
|||
|
Signet ring cell |
1.3 |
(0.7–2.4) |
0.401 |
|
|
Nonmetastatic disease (DFS) |
||||
|
Lymph node ratio |
||||
|
≤0.6 |
1 |
|||
|
> 0.6 |
2.83 |
(0.7–11.5) |
0.145 |
|
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
Discussion
Gastric cancer is a disease of old age. The mean age for gastric cancer ranges between 50 and 70 years. The combination of environmental factors and accumulation of various genetic alterations after long period of atrophic gastritis are supposed to be reason predilection for older age. Consistent with the epidemiologic data worldwide, the prevalence of gastric cancer in young patients (<30 years) in present study found prevalence as 2.5%. Chronic atrophic gastritis primarily associated with chronic Helicobacter pylori infection and subsequent intestinal metaplasia leads to intestinal-type cancers.8,9 Younger patients have higher proportion of the diffuse gastric cancer probably because they have fewer years to develop intestinal metaplasia. Environmental toxins play lesser role in younger patients.
One of the major factors involved in development of gastric cancer in younger patients is genetic predisposition due to the presence of CDH1 mutation.10,11 Germline CDH1 mutations encode aberrant form of E-cadherin, resulting in hereditary diffuse cancer.12,13 In present study, 14.2% of the young gastric patients had a positive family history of gastric cancer and other 10% patients gave history of either uterine cancer, colon cancers, and sarcomas. This incidence is lesser than reported by others,14 that is, 17 to 25%. Probable reason may be due to lower rate of literacy than that of developed countries and inability to recall the disease of their relatives.
Consistent with the location described by Theuer et al in their population-based registry, 35% of our patients had growth at antral region.7 We currently do not know the reason for this predilection. Many of the other authors have described similar findings.15 The most common variety were poorly differentiated adenocarcinoma and signet ring cell carcinoma in these patients probably explaining the reason as to why 70% of our patient presented in stage IV at diagnosis. However, other factors may be operating as well. Our study has found that young gastric cancer patients are two times likely to be male which is opposite of what other studies from other Asian countries have found.15 This difference may be probably related to complex interplay between toxins exposure, genetic makeup, and hormonal differences.
D2 lymphadenectomy with gastrectomy was performed in all patients who underwent curative resection for the locoregional disease. The median OS was 9.2 and 21.6 months, respectively, in metastatic and locoregional disease clearly demonstrating the need for early detection and management. Different studies have shown nodal ratio (NR) as an independent prognostic factor regardless of the type of lymphadenectomy performed and the number of lymph nodes removed during the surgical procedure.16,17,18,19,20,21,22 NR, defined as ratio between the number of metastatic nodes and the number of examined nodes, has been proposed as a valuable and independent prognostic factor in patients with gastric cancer after D2 and also D1 dissection. Presently, there is no consensus regarding the most appropriate cutoffs for NR and several different NR cutoffs have been used in different studies. Moreover, none of studies have reported outcome in young patients according to NR. In the present study, LNR more than 0.6 had strong trend toward worse survival with HR of 2.83, although not statistically significant probably because of small sample size of group undergoing curative surgery. This is thought provoking and needs further investigation.
The various studies have consistently reported poor OS in young gastric cancer patients as compared with older adults.23,24,25 In our study, the median OS in stage IV disease was 9.2 months, while median DFS in those treated with curative intent was 10.5 months. Diagnostic delays and the more aggressive biology of gastric cancer in younger patients are usually suggested as possible reason for poorer outcome. Survival difference was not seen between males and females or between different histologies (signet vs non signet ring). Trend toward better survival was also seen in patients younger than 20 years as compared with less than 20 years in present study. In our present study, the most common site of metastasis was peritoneum followed by liver. The overall lower survival rate is less than Japan but comparable to rest of the world probably because percentage of early gastric cancer patients in our study was very low.
Tendency of late diagnosis of gastric cancer is seen in younger patients. Physicians should have a high index of suspicion of the disease in patients with a family history suggestive of cancer, with ulcer symptoms that do not improve on medical management, or once an ulcer is diagnosed. In addition, upper gastrointestinal endoscopy with biopsy should be carried at earliest for early diagnosis, which may increase the survival of young gastric cancer patients.
Conclusion
Incidence of stomach cancer in young patient is more than expected and more than global average in India. Most of these young patients are presenting in advanced stage and survival is poor compared with typical aged patients. Large studies to find out the causes of gastric cancer in young and adequate interventions to bring awareness and early diagnosis are important to improve survival.
Conflict of Interest
None declared.
References
- Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(06):425-445.
- [Google Scholar]
- The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(01):42-54.
- [Google Scholar]
- Epidemiological review of gastric cancer in India. Indian J Med Paediatr Oncol. 2011;32(01):3-11.
- [Google Scholar]
- Unique features of gastric carcinoma in the young: a population-based analysis. Cancer. 1998;83(01):25-33.
- [Google Scholar]
- Analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol Oncol Res. 2013;19(01):111-117.
- [Google Scholar]
- Clinicopathological comparison between young and old age patients with gastric adenocarcinoma. Int J Gastrointest Cancer. 2005;35(01):43-52.
- [Google Scholar]
- Gastric cancer: new genetic developments. J Surg Oncol. 2005;90(03):114-133. , discussion 133
- [Google Scholar]
- Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med. 2001;344(25):1904-1909.
- [Google Scholar]
- Characterization of a recurrent germ line mutation of the E-cadherin gene: implications for genetic testing and clinical management. Clin Cancer Res. 2005;11(15):5401-5409.
- [Google Scholar]
- Clinicopathologic characteristics of gastric cancer in a young patient population. J Gastrointest Surg. 2004;8(03):240-244.
- [Google Scholar]
- Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer. 2018;21(06):889-899.
- [Google Scholar]
- Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96(02):95-101.
- [Google Scholar]
- The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9(01):27-34.
- [Google Scholar]
- The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245(04):543-552.
- [Google Scholar]
- Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10(09):1077-1085.
- [Google Scholar]
- Prognostic impact of metastatic lymph node ratio on gastric cancer after curative distal gastrectomy. World J Gastroenterol. 2010;16(16):2055-2060.
- [Google Scholar]
- Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol. 2008;97(02):132-135.
- [Google Scholar]
- Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16(02):319-326.
- [Google Scholar]
- Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. 2010;16(02):256-263.
- [Google Scholar]
- Clinicopathologic characteristics and prognosis for young gastric adenocarcinoma patients after curative resection. Ann Surg Oncol. 2008;15(05):1464-1469.
- [Google Scholar]
- Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg. 1996;172(05):473-476. , discussion 476–477
- [Google Scholar]







