Assessment of swallowing dysfunction using fees (flexible endoscopic evaluation of swallowing) in head and neck cancer patients undergoing radiotherapy
Corresponding author: Aathirai Mahendiran, Department of Radiation Oncology, Apollo Cancer Centre, Teynampet, Chennai, India. aathirai92@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mahendiran A, Potharaju M, Chandrasekhar S. Assessment of swallowing dysfunction using fees (flexible endoscopic evaluation of swallowing) in head and neck cancer patients undergoing radiotherapy. Asian J Oncol. 2024;10:4. doi: 10.25259/ASJO_26_2024
Abstract
Objectives
The aim of this study is to evaluate swallowing dysfunction at baseline (before radiotherapy), at one month and three months post-radiotherapy and to assess time taken for these parameters to come back to normal in head and neck cancer patients.
Material and methods
Total 30 patients who received radiotherapy for head and neck cancer, either as inpatients or outpatients, at Apollo Speciality Hospital, Chennai from June 2022 and May 2023 (1 year). Laryngeal sensation and pharyngeal swallowing before radiotherapy, at one month and three months post-radiotherapy assessed using penetration aspiration scale (PAS Scale), bolus residue scale (BRS Scale) and subjective assessment using eating assessment tool-10 (EAT-10) questionnaire and assess time it take to come to normal
Results
The EAT-10,PAS and BRS scores were analysed by multivariate analysis. All these scores showed a definite improvement from baseline (Before Radiotherapy) to three months Post-RT. Further more our study predicts the hazard ratio, through which the exact percentage and number of days of one variable compared with other variable, to come back to normal can be predicted. EAT-10 scores showed the influence of age and RT dose. More the age and higher the dose more time is taken for the scores to return back to normal. In PAS and BRS males took a longer time for the scores to come back to normal, whereas patients with >60 years of age (categorical) and every year increase in age (Continuous) took a longer time to return back to normal of PAS and BRS scores respectively.
Conclusion
It is possible to predict the number of days it takes for the EAT-10, PAS and BRS score to return back to normal. So that we can initiate preventive measures like swallowing exercises, nutritional advise and Ryles tube insertion at the earliest for those who may develop swallowing complications, to enhance the quality of life for these patients.
Keywords
Swallowing dysfunction
Eating Assessment Tool-10
Flexible Endoscopic Evaluation of Swallowing
Head and neck cancer
INTRODUCTION
With an annual toll of 1.1 million, head and neck cancer ranks as the seventh most prevalent cancer worldwide.[1] Standard treatments such as radiotherapy (RT) and concurrent chemoradiotherapy (CCRT), vital for locally advanced cases, often introduce debilitating side effects, notably dysphagia, significantly impacting patients’ quality of life (QOL).[2,3] In the challenging landscape of inoperable head and neck cancer, CCRT emerges as the primary therapeutic approach, albeit accompanied by the discordant notes of high toxicity, particularly affecting critical swallowing structures and resulting in dysphagia.[4]
Dysphagia, stemming from both cancer and treatments, induces intricate anatomical and functional alterations, significantly diminishing QOL. Swallowing impairment, often underestimated, emerges as a post-RT concern, with silent aspiration posing threats to weakened patients.[5]
For those traversing the realms of RT or CCRT, the journey involves an elevated risk of dysphagia, a severe side effect casting its shadow both in early and late stages.[5] Prevalent in 40% of cases post-RT, dysphagia unveils life-threatening risks, including aspiration, encompassing anatomical and functional alterations that complicate swallowing, leading to reduced food intake.[6] The often-overlooked consequence of swallowing impairment can culminate in silent aspiration, underscoring the imperative for objective assessments.[7]
Objective assessments are imperative for posttreatment dysphagia evaluation. Techniques such as video fluoroscopic swallow study (VFSS) and flexible endoscopic evaluation of swallowing (FEES) offer nuanced insights.[8] VFSS, a modified barium swallowing examination, provides real-time visualization, aiding in identifying aspiration causes and effects of different bolus volumes and textures.[9] FEES, a preferred method for its ease, tolerability, bedside examination, and cost-effectiveness, allows direct visualization of the swallowing process.[10]
Our study takes a comprehensive approach, gauging swallowing subjectively via the eating assessment tool-10 (EAT-10) score and objectively through FEES. The EAT-10, a validated tool, captures self-reported difficulties, while FEES facilitates real-time observation, delving into structural and functional aspects. This dual-pronged approach seeks a nuanced understanding of how dysphagia resonates in the realm of swallowing function, offering valuable insights into its profound impact.[7]
OBJECTIVES
Primary
-
I.
To evaluate the laryngeal sensation and pharyngeal swallowing using:
-
Penetration aspiration scale (PAS Scale)
-
Pharyngeal bolus residue scale (BRS Scale)
-
Subjective assessment using EAT-10 questionnaire.
Values will be taken before RT, at one month post-RT, and at three months post-RT.
-
-
II.
To assess the time taken for these parameters to come back to normal.
Secondary
To assess factors like patient characteristics, tumor characteristics, and treatment characteristics that influence the parameters like eating assessment tool-10 (EAT-10), penetration aspiration scale (PAS), and bolus residue scale (BRS) to come back to normal.
MATERIAL AND METHODS
A prospective observational study was conducted at our hospital in the Deglutition and Swallowing Suite from June 2022 to May 2023. Approval was obtained from the Scientific Research and Ethical Committee of our hospital. Informed consent was obtained from the participants before their involvement in the study.
Adults with confirmed squamous cell carcinoma or adenocarcinoma in the head and neck region, cancers where the treatment portal includes the pharyngeal region, aged 18 and above, were eligible. Inclusion required nonmetastatic disease, eastern cooperative oncology group (ECOG) performance status of I or II, and informed consent. Exclusions applied to cases with disease recurrence, metastasis, prior head and neck irradiation, or radiotherapy (RT) portals excluding the pharyngeal region. Patients with tracheostomy or Ryles tube were ineligible.
Collected demographic and baseline data at entry: age, sex, primary cancer site, tumour, node, metastasis (TNM) classification, total radiation dose, RT technique, and chemotherapy regimen. Preradiation assessment included eating assessment tool-10 questionnaire and objective analysis of penetration aspiration scale and bolus residue scale via FEES procedure. Simulation and planning computed tomography was performed for radiation therapy to contour, plan, and evaluate the RT treatment. Post-RT assessments at one and three months were done using EAT-10, PAS, and BRS via FEES, noting the time for scores to normalize. Data were compiled and analyzed in excel. Statistical analysis was conducted for interpretation and conclusions.
Statistical Analysis
Descriptive statistics (frequency, mean/standard deviation, median/inter quartile range for nonnormally distributed data) will be used. Normality will be checked with the Shapiro-–Wilk test. Associations between categorical factors will be tested using Chi-square/Fisher’s exact tests. Changes in continuous factors over time will be assessed with paired t-tests/Wilcoxon Sign Rank tests. Cox regression analysis will identify independent variables affecting dependent variables. Univariate/multivariate Cox proportional hazard models will estimate hazard ratios and 95% confidence intervals (CIs). Hazard ratios quantify the correlation. Log minus log plot checks the proportional hazard assumption. Data entry will be done in Excel, and analysis will be performed with statistical package for the social sciences (SPSS) (Version 27.0). Significance is set at p < 0.05. This concise plan guides systematic data analysis and interpretation.
Tools used for analysis
EAT-10 (Eating assessment tool) questionnaire is used for subjective analysis.[11] FEES procedure was carried out by SLP technician in our hospital in the deglutition and swallowing suite.
In the FEES procedure, equipment was prepared, and the patient’s consent was obtained. After applying local anesthesia, a lubricated endoscope was inserted through the nostril for real-time visualization of the pharynx, larynx, and upper esophageal sphincter. Swallowing trials with various consistencies were conducted, and the entire procedure was recorded [Figures 1 and 2].

- Fiberoptic endoscopic evaluation of swallowing (FEES) instruments – entire setup.
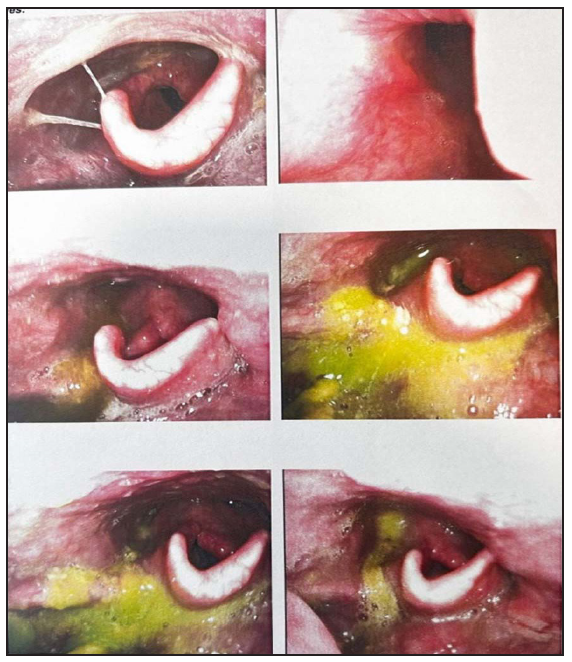
- Fiberoptic endoscopic evaluation of swallowing (FEES) final report showing penetration for liquids with bolus residue.
Analysis included PAS[12] and BRS[13] scoring, providing insights for management recommendations like dietary adjustments.
RESULTS
A total of 41 patients were enrolled: two were lost to follow-up, one patient had Ryles tube insertion done during RT, and one patient deteriorated in the course of RT. So, finally, 37 patients were taken for analysis [Table 1].
| Patient characteristics | Categories | Total cases (37) |
|---|---|---|
| Age | <40 | 6 |
| 40–60 | 24 | |
| >60 | 7 | |
| Gender | Male | 22 |
| Female | 15 | |
| Performance Status | 1 | 20 |
| 2 | 17 | |
| Stage | I | 3 |
| II | 11 | |
| III | 14 | |
| IV | 9 | |
| Chemotherapy | Yes | 26 |
| No | 11 | |
| RT dose | 66 Gy | 24 |
| 70 Gy | 13 | |
| RT technique | IMRT | 18 |
| VMAT | 19 |
RT: Radiotherapy, Gy: Gray, IMRT: Intensity modulated radiotherapy, VMAT: Volumetric modulated arc therapy.
We used Cox regression models to predict the time for eating assessment tool, penetration aspiration scale, and bolus residue scale scores to return to mild to normal range after RT or concurrent chemoradiation. We utilized univariate and multivariate Cox proportional hazards models, considering a p-value < 0.05 as statistically significant. Due to a one year study period, follow-up was limited to six months, with event/censored data considered until 120 days for parameters to return to normal.
Results based on normalization of the scores witgh respect to the covariates such as age, gender, performance status,diagnosis, staging, radiotherapy details and baseline scores. Cox regression evaluated age, eating assessment tool at baseline (EATBASE), penetration aspiration scale at baseline (PASBASE), bolus residue scale at baseline (BRS) as both continuous and categorical variables, while other covariates were considered categorical. The time for the scores to return back to normal has been recognized as a surrogate measure of the quality of life, indicating that less time for scores to normal leads to improved swallowing capability and better patient quality of life.
Time taken for eating assessment tool (EAT) score to return to normal as a dependent variable (“EATTIME”)
Here, we have a likelihood ratio of the fit of the full model relative to a null (intercept only) model. Statistical significance suggests that the model is a significant improvement [χ 2[12] = 37.585, p < 0.001] in fit relative to the null. This indicates that at least one population regression coefficient is different from 0. A log minus log plot for the variables of interest is usually plotted to check whether the proportional hazards assumption holds good and is not violated.
In Figure 3, we can see that the lines are parallel to one another for the variable “Age Categorical”, age as a categorical variable and hence the proportional hazards assumption holds good. This was checked for all variables of interest to make sure that the proportional hazards assumption was not violated.
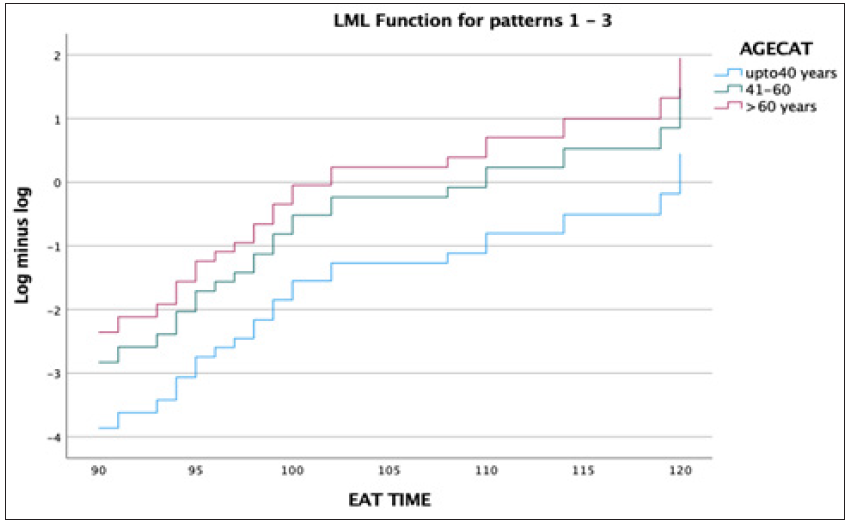
- Log minus log of EAT TIME – Age Cat. EAT: Eating assessment tool, AGECAT: Age categorical, LML: Log minus log.
In Table 2, hazard ratio for EATBASE C CATEGORIAL (event vs censored) is 0.079 (95% CI, 0.01–0.52; P = 0.009), signifying a 7.9% longer time for eating assessment tool score normalization if the baseline is moderate to severe (statistically significant). hazard ratio for eating assessment tool at baseline continuous is 0.82 (95% CI, 0.71–0.95; P = 0.01), indicating 17.2 days increase for EATSCORE normalization with a 1-point increase (statistically significant).
| Variables |
Hazard ratio (95% CI) |
P-Value |
|---|---|---|
| Nasopharynx and oral cavity | 0.49 | |
| Oropharynx and larynx |
2.29 (0.40–12.95) |
0.34 |
| Hypopharynx |
1.07 (0.22–5.10) |
0.92 |
| Stage |
0.80 (0.27–2.33) |
0.68 |
| RT technique |
1.09 (0.41–2.93) |
0.85 |
| RT dose |
3.27 (0.98–10.92) |
0.05 |
| Chemotherapy |
1.40 (0.47–4.15) |
0.54 |
| Age continuous |
0.89 (0.81–0.98) |
0.02 |
| Age cat (0), <40 | 0.73 | |
| Age cat (1), 41–60 |
0.22 (0.004–12.43) |
0.46 |
| Age cat (2), >60 |
0.62 (0.08–4.39) |
0.63 |
| Gender (Male vs Female) |
2.27 (0.81–6.36) |
0.11 |
| Eat base (Categorical) |
0.07 (0.01–0.52) |
0.009 |
| Eat base continuous |
0.82 (0.71–0.95) |
0.01 |
RT: Radiotherapy, CI: Confidence interval, Age cat: Age categorical, Eat: Eating assessment tool, Bold value indicates its statistically significant.
Hazard ratio for age continuous is 0.89 (95% Confidence Interval, 0.81–0.98; P = 0.02), implying 10.4 days increase for EAT normalization with a one year age increase (statistically significant). Radiotherapy dose as a categorical variable (0=66 Gray, 1=70 Gray) has HR 3.27 (95% Confidence Interval, 0.98–10.92; P = 0.05), suggesting 3.28 times longer for EAT scores to return to normal with 70 Gy compared to 66 Gy (marginally significant). Other variables like types of cancer, stage, chemotherapy, performance status, and RT technique were not statistically significant.
Time taken to penetration aspiration scale (PAS) score to return to normal as a dependent variable (“PASTIME”)
Table 3 shows that the HR for gender was 4.55 (95% CI, 1.44-14.37; P = 0.01). This is interpreted as it takes 4.6 times more number of days for penetration aspiration scale score to come to the normal range in males compared to females. The hazard ratio for “AGECAT” variable (age as categorical variable) was 0.18 (95% CI 0.04–0.820; P = 0.02), i.e., it takes 8.9% less number of days for PAS score to come back to normal after treatment in the age category 41–60 years compared to patients > 60 years group. None of the other variables were statistically significant.
| Variables |
Hazard ratio (95% CI) |
P-Value |
|---|---|---|
| Nasopharynx and oral cavity | 0.61 | |
| Oropharynx and larynx |
1.27 (0.40–4.01) |
0.67 |
| Hypopharynx |
1.82 (0.53–6.19) |
0.33 |
| Stage |
0.94 (0.40–2.23) |
0.90 |
| RT technique |
1.007 (0.38–2.62) |
0.98 |
| RT dose |
0.60 (0.22–1.61) |
0.31 |
| Chemotherapy |
1.48 (0.57–3.83) |
0.41 |
| Age continuous |
0.96 (0.88–1.04) |
0.33 |
| Age cat (0), <40 | 0.08 | |
| Age cat (1), 41–60 |
0.08 (0.005–1.38) |
0.08 |
| Age cat (2), >60 |
0.18 (0.04–0.82) |
0.02 |
| Gender (Male vs Female) |
4.55 (1.44–14.37) |
0.01 |
| PAS base categorical |
1.48 (0.31–6.92) |
0.61 |
| PAS base continuous |
0.79 (0.49–1.27) |
0.33 |
RT: Radiotherapy, CI: Confidence interval, Age cat: Age categorical, PAS: Penetration aspiration scale, Bold value indicates its statistically significant
Time taken to bolus residue scale (BRS) score to return to normal as a dependent variable (“BRSTIME”)
The HR for “AGE CONTINUOUS”, i.e., age as a continuous variable was 1.12 (95% CI, 1.009–1.24; P = 0.03). There is an increase by 1.12 times in the number of days required for bolus residue scale score to come to normal range for every 1-year increase in age. The HR for “GENDER” was 3.58 (95% CI, 1.07–11.90; P = 0.03). The HR for “Bolus Residue Scale at Baseline” is 0.51 (95% CI, 0.30–0.86; P =0.01). It takes 48.4 % more number of days for the BRS score to come to normal for every unit increase in the BRS score [Table 4]. None of the other variables showed any statistical significance.
| Variables |
Hazard ratio (95% CI) |
P-Value |
|---|---|---|
| Nasopharynx and oral cavity | 0.88 | |
| Oropharynx and larynx |
0.73 (0.20–2.65) |
0.63 |
| Hypopharynx |
0.72 (0.17–2.95) |
0.65 |
| Stage |
0.73 (0.30–1.74) |
0.47 |
| RT technique |
1.02 (0.42–2.47) |
0.95 |
| RT dose |
0.44 (0.17–1.11) |
0.08 |
| Chemotherapy |
2.14 (0.72–6.35) |
0.16 |
| Age continuous |
1.12 (1.009–1.24) |
0.03 |
| Age cat(0), <40 | 0.12 | |
| Age cat(1), 41–60 |
4.93 (0.19–126.75) |
0.33 |
| Age cat (2), >60 |
0.87 (0.15–4.87) |
0.87 |
| Gender (Male vs Female) |
3.58 (1.07–11.90) |
0.03 |
| BRS base continuous |
2.00 (0.41–9.79) |
0.38 |
| BRS base continuous |
0.51 (0.30–0.86) |
0.01 |
RT: Radiotherapy, CI: Confidence interval, Age cat: Age categorical, BRS: Bolus residue scale, Bold value indicates its statistically significant
Time for the score to come back to normal – event/censored
Since our study period is one year, we were able to follow a few patients who were recruited earlier in the study for around six months. The event/censored data for the scores to revert back to normal were taken at 120 days post-RT. Tables 5, 6, and 7 reveal the time taken for EAT-10, PAS, and BRS to revert back to normal, respectively.
| EAT-10 | Event/Censored | Number | Percent |
|---|---|---|---|
| Event | 27 | 73 | |
| Censored | 10 | 27 | |
| Total | 37 | 100 |
EAT-10: Eating assessment tool-10.
| PAS | Event/Censored | Number | Percent |
|---|---|---|---|
| Event | 31 | 83.8 | |
| Censored | 6 | 16.2 | |
| Total | 37 | 100 |
PAS: Penetration aspiration scale.
| BRS | Event/Censored | Number | Percent |
|---|---|---|---|
| Event | 28 | 75.7 | |
| Censored | 9 | 24.3 | |
| Total | 37 | 100 |
BRS: Bolus residue scale.
The baseline to one month category and one month to three month category analysis was done. Since it did not show any statistical significance, they were not projected in the paper.
DISCUSSION
Head and neck cancer is globally recognized as the seventh most prevalent form of cancer. For patients with locally advanced head and neck cancers, radiotherapy or concurrent chemo-radiotherapy has emerged as the established standard of care, offering improved outcomes in terms of local control and overall survival.[1] Swallowing impairment frequently follows RT for head and neck malignancies, significantly impacting nutrition and overall quality of life (QOL).[14]
Logemann et al. emphasize that pretreatment dysphagia severity is influenced by tumor stage and site, with significant impacts on specific swallowing disorders.[15] Liou et al. stress the need for combined objective and subjective dysphagia assessments, highlighting varying outcomes in oropharyngeal and nasopharyngeal tumors. They identify advanced age, multiple head and neck cancer (HNC) diagnoses, and radiotherapy as predictors of unfavorable scores.[16] Xinou et al.’s study on HNC patients post-chemo-radiotherapy (CRT), using video fluoroscopic swallow study (VFSS) and modified barium swallow impairment profile (MBSImP), underscores persistent severe swallowing deficits and aspiration risks, urging comprehensive evaluations for better understanding.[17]
Dysphagia and aspiration are recognized as fatal side effects. These factors all pointed to the requirement for an objective analysis of these functions. This was evaluated using the fiberoptic endoscopic evaluation of swallowing (FEES) technique. In our study, the main objective of the study is to assess the swallowing subjectively and objectively.
The eating assessment tool-10, penetration aspiration scale, and bolus residue scale scores were analyzed statistically. All these scores showed a definite improvement from baseline (Before Radiotherapy) to three months post-radiotherapy. Furthermore our study predicts the hazard ratio, from which we can predict the exact percentage and number of days the scores to come back to normal, with one variable compared to the other.
After multivariate statistical analysis, radiation dose and age were of statistical significance as continuous variables in the eating assessment tool-10 score. For every year increase in age, the duration of time taken for EAT-10 to come back to normal increased (approx. 10.4 days). Patients who received 70 Gray took longer time (approx. 3.28 times) to come back to normal than who received 66 Gray.
Similarly, in multivariate statistical analysis for penetration aspiration scale scores, gender and age were of significance as categorical variables. Males took a longer time (approx. 4.6 times) compared to females and patients in the category 41–60 years of age took less number of days (8.9% less) for the scores to return to normal compared to >60 years age.
In the bolus residue scale category, age and gender were of statistical significance. For every year increase in age, it took a longer time for the BRS score to come back to normal (1.12 times). BRS score for males (3.5 times more) took a longer time to revert back to normal. Dysphagia/Aspiration at risk structures were contoured and analyzed in both the techniques volumetric modulated arc therapy (VMAT) and intensity modulated radiotherapy (IMRT). Since it did not show statistical significance, it was not depicted in our thesis. Similarly, the baseline to one month category and one month to three month category analysis was done. Since they did not show any statistical significance, they were not projected in the paper.
Limitations of the study
The study had one year duration, but longer follow-up periods would have been beneficial to assess treatment outcomes and long-term effects, considering that some patients may require extended monitoring. The study’s small sample size limited the statistical analysis and the generalizability of the findings.
Future directions – shaping the future of dysphagia management
This study was only an observational study. In future, a comparative study can be conducted with Observation on the one hand and Therapeutic group on the other, where the effect of swallowing after administering swallowing exercises can be determined.
These patients can be followed up further more years to find exactly when they return back to normal scores.
This study if followed for further more years and with a good sample size can form a basis of AI (Artificial Intelligence) predictive swallowing models which might actually predict the amount of swallowing difficulty which a patient will develop for a particular age, sex, performance status (PS), site of tumor, and stage of tumor with various techniques used.
CONCLUSION
In our model, it is possible to predict the number of days it takes for the eating assessment tool-10, penetration aspiration scale, and bolus residue scale scores to return back to normal. We can identify these subsets of patients who are prone to develop complications like swallowing difficulties and aspiration so that we can initiate early preventive measures like swallowing exercises, nutritional advises, and Ryles tube insertion at the earliest. By implementing these measures earlier in the treatment process, we can enhance the quality of life for these patients, thereby improving their overall well-being and functional outcomes.
Acknowledgement
SLP technician : Ms. Janani
Department of Radiation Oncology :
Consultants : Dr. Rathna Devi, Dr. Shankar Vangipuram, Dr. Rajendran, Dr. Subathira, Dr. Bhargavi Elangovan, and Dr. Shakespeare.
Radiation Physicist & Radiation Technologist
Dr. Muralidharan. M : Director of Medical Education, Apollo Hospitals, Chennai
My friends, parents and family
Ethical approval
The research/study was approved by the Institutional Review Board at Apollo Hospital AHEL, Chennai, number ASH-DNB-026/06-22, dated 25.06.2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Competing morbidities in advanced head and neck squamous cell carcinoma concurrent chemoradiotherapy: A strong implication of a multidisciplinary team approach. Cancer Manag Res. 2019;11:9771-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: Cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An updated review on head and neck cancer treatment with radiation therapy. Cancers (Basel). 2021;13:4912.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tennant: Pathophysiology of radiation-induced dysphagia in head and neck cancer. Dysphagia. 2016;31:339-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anatomy and physiology of feeding and swallowing-normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691-707.
- [CrossRef] [PubMed] [Google Scholar]
- Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fiber-optic endoscopic evaluation of swallowing (FEES): predictor of swallowing-related complications in the head and neck cancer population. Head Neck. 2013;35:974-9.
- [CrossRef] [PubMed] [Google Scholar]
- The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19:769-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fiberoptic endoscopic evaluation of swallowing (FEES): Proposal for informed consent. Acta Otorhinolaryngol Ital. 2008;28:206-11.
- [PubMed] [PubMed Central] [Google Scholar]
- Validity and reliability of the eating assessment tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117:919-24.
- [CrossRef] [PubMed] [Google Scholar]
- Scoring the penetration–aspiration scale (PAS) in two conditions: A reliability study. Dysphagia. 2022;37:407-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bolus residue scale: An easy-to-use and reliable videofluoroscopic analysis tool to score bolus residue in patients with dysphagia. Int J Otolaryngol. 2015;2015:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Assessment of voice, speech, and related quality of life in advanced head and neck cancer patients 10-years+ after chemoradiotherapy. Oral Oncol. 2016;55:24-30.
- [CrossRef] [PubMed] [Google Scholar]
- Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64-73.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of objective and subjective swallowing outcomes in patients with dysphagia treated for head and neck cancer. J Clin Med. 2022;11:692.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Longitudinal evaluation of swallowing with VFSS in patients with locally advanced head and neck cancer after chemoradiation. Dysphagia. 2018;33:691-706.
- [CrossRef] [PubMed] [Google Scholar]







