Translate this page into:
Assessment of Celiac Axis and Hepatic Artery Variations in Hepatobiliary and Pancreatic Malignancy with Multidetector Computed Tomography Angiography
Address for correspondence Namrata Kaushik, MBBS, MD, Department of Radiodiagnosis, Dr. Ram Manohar Lohia Institute of Medical Sciences, Gomti Nagar, Vibhuti Khand, Lucknow 226010, Uttar Pradesh, India. namratak5867@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction Delineation of variant anatomy in celiac trunk and superior mesenteric artery including its origin and branching pattern and variations in branching pattern of hepatic artery in patients of hepatopancreaticobiliary malignancy with the use of mutidetector CT (computed tomography) angiograpgy was performed.
Materials and Methods All CT examinations were performed on a 64-multidetector computed tomography (MDCT) scanner. Technical features of multislice computed tomography (MSCT) are as follows: 64 mm × 1 mm collimation, minimum slice thickness of 0.625, gantry rotation time of 320 ms, 120 kV, and 320 mAs. CT angiography was performed with intravenous (IV) administration of nonionic contrast material, that is, iodixanol (Visipaque). The contrast medium and saline solution were injected with a Medrad power injector at 3 mL/sec through an 18-gauge plastic intravenous catheter placed in an antecubital vein in most of the cases. Contrast medium volumes varied between 100 and 150 mL at 1.5 mL/kg. Images were obtained in triphasic pattern at arterial (20–30 seconds), portal (60–70 seconds), and equilibrium (at 3 minutes) phases.
Results Five types of celiac axis anatomic variations and nine type of variants in celiac axis branching was found in the study sample of 124 patients. Classical celiac axis anatomy was seen in 92.7% of the cases, while the five types of variation in branching were found in nine patients. Majority of cases showed pattern I (59.6%) followed by patterns V (12.1%), II (9.7%), and III (8.9%). There were three (2.4%) cases each showing pattern VIII and AA, and two (1.6%) cases each showing patterns IV and VI, respectively. There was one (0.8%) case each showing pattern VII and IX. A total of three (2.4%) cases showed right hepatic artery arising from celiac axis.
Conclusion We conclude that most common pattern of celiac axis and superior mesenteric artery (SMA) branching is classical pattern (92.7%) which is in concordance with literature. Type-I pattern of hepatic artery branching was most common (59.6%), similar to that documented in literature. Although the most common variation in our study is type V (12.1%), followed by types II (9.7%) and III (8.9%), the most common variation in most of the literature was found to be type III. CT angiography hence is an excellent diagnostic modality for depiction of arterial anatomic variations and provides a roadmap for surgical treatment.
Keywords
multidetector computed tomography
CT angiography
arterial variants
celiac axis
superior mesenteric artery
hepaticopancreatobiliary malignancy
Introduction
Multidetector computed tomography (MDCT) has revolutionized vascular imaging with CT angiography, giving the same information as conventional angiography noninvasively. CT angiography (CTA) is often performed in patients with pancreatic and hepatobiliary neoplasms to determine tumor resectability.
Surgery is regarded as the only definitive treatment for most hepatic, pancreatic, and biliary malignancies; more favorable in early stages of the disease, and radical resection is performed even for the advanced stage of the tumor. Complete preoperative evaluation remains the key factor for successful surgery in all hepatobiliary and pancreatic malignancies.
MDCT enables the rapid acquisition of thin-slice high-resolution images of the abdominal arteries during the phase of maximal contrast enhancement. The volumetric acquisition of data allows three-dimensional (3D) reconstructions to be created, providing the surgeon with a 3D model of the patient’s arterial anatomy.1 This can be of great value because direct visualization of the surgical field is often limited in patients with pancreatic and hepatobiliary malignancy.
In addition to evaluating for vascular involvement by tumor, it is also important for the radiologist to assess for the presence of variant arterial anatomy. Accurate evaluation of the segmental anatomy and development of a road map of the arterial anatomy are essential prerequisites for planning of the surgeries.
The presence of different arterial variants may alter patient management. Therefore, having an awareness of the CTA appearance of these variants and an appreciation of their clinical significance is of great importance.2
The aim of this study was to determine the frequency of different arterial variants identified at abdominal CTA performed in a patient diagnosed with pancreatic or hepatobiliary malignancy.
We did not intend to assess the sensitivity or specificity of CTA in detecting arterial variants, which have already been reported,3,4 but rather to show that the broad range of common and clinically significant arterial variants is visible using CTA.
Although there have been previous studies describing arterial variants, this study is among the few to be conducted among the north Indian population, analyzing both vascular variants in hepatobiliary and pancreatic malignancies. Because CTA is becoming more widely used in the evaluation of patients with hepatic and pancreatic neoplasm, recognition of both the CT appearance, and the clinical significance of these variants is essential for patient management.
Materials and Methods
The study was done at department of Radiodiagnosis Dr. Ram Manohar Lohia Institue of Medical Sciences (RMLIMS) in Lucknow between January 2018 and June 2019 and included 128 patients diagnosed and suspected with hepatopancreaticobiliary malignancy. CTA was performed to determine tumor resectability and to aid surgical planning. Each patient was included only once in the study. Patient with history of abdominovascular or pancreatic surgery or with deranged renal function or having any kind of contraindication for the use of iodinated contrast media were excluded from the study.
Our institutional review board approved the study and patient informed consent was obtained.
CT examination protocol—triphasic CT angiography: all CT examinations were performed on a 64-MDCT scanner (Philips Medical System Version 6.4, Extended Brilliance Workspace). Technical features of multislice computed tomography (MSCT) are as follows: 64 mm × 1 mm collimation, minimum slice thickness of 0.625, gantry rotation time of 320 ms, 120 kV, and 320 mAs. The patients did not eat or drink for 4 to 6 hours. After nonenhanced liver images, CTA was performed after the intravenous (IV) administration of nonionic contrast material (iodixanol [Visipaque]). The contrast medium and saline solution were injected with a Medrad power injector at 3 mL/sec through an 18-gauge plastic intravenous catheter placed in an antecubital vein in most of the cases. Contrast medium volumes varied between 100 and 150 mL at1.5 mL/kg. Water (∼2,000 mL) was administered to all patients as a nonopaque oral contrast agent. Dynamic images were obtained during the hepatic arterial, portal venous, and equilibrium phases. After injection of intravenous contrast material, liver was scanned in arterial (scanning delay, 20–30 seconds), portal (scanning delay, 60–70 seconds), and equilibrium (scanning delay, at 5 minutes) phases.
Image Interpretation
MDCT images were processed using a various technique such as multiplanar reformation, maximum intensity projection, and volume rendering at the available workstation.
Nomenclature System and Classification for Origin and Variants of Arteries
The current textbook definition of classical celiac axis is an arterial trunk originating from the aorta to the branching point of the common hepatic artery (CHA) and splenic artery (SpA), with the left gastric artery (LGA) being the first branch stemming from the arterial trunk.5 In reality, however, the LGA, CHA, and SpA can all have variant origins other than the celiac axis. The currently accepted definition of celiac axis, therefore, does not allow for detailed description of celiac axis variations including variant origins of its three major branches. Therefore, the celiac axis has been redefined as an arterial trunk containing at least two of its major branches. The CMT as an arterial trunk containing the superior mesenteric artery (SMA) and at least two major branches of the celiac axis. According to the above-mentioned definition of the celiac axis and CMT, following 15 possible variations in branching pattern of celiac axis and SMA are possible6:
Celiac axis variations:
-
Hepatosplenic trunk + LG + SM
-
Hepatomesenteric (HM) trunk + gastrosplenic (GSp) trunk
-
Celiomesenteric (CM) trunk
-
HSpM trunk + LG
-
HM trunk + LG + Sp
-
CH + GSp trunk + SM
-
HG trunk + SpM trunk
-
CH + LG + Sp + SM
-
CH + GSpM trunk
-
CH + LG + SpM trunk
-
HG trunk + Sp + SM
-
HSp trunk + GM trunk
-
HGM trunk + Sp
-
CH + GM trunk + Sp
-
Ambiguous anatomy
In our study, classification of celiac axis and SMA origin variants have been done into the six groups found in the study (Table 1).
|
S. No. |
Groups |
Description |
|---|---|---|
|
1. |
Classical |
CA and SMA arising separately from the aorta, and the CA gives rise to CHA, SpA, and LGA |
|
2. |
LGA + HSp + SMA |
Hepatosplenic trunk with LGA and SMA arising from the aorta |
|
3. |
LGA + CHA + SpA + SMA |
CHA, LGA, SpA and SMA arising separately from the aorta |
|
4. |
CM Trunk |
Celiomesenteric trunk, an arterial trunk containing the SMA and at least two branches. |
|
5. |
GSp + HM |
Gastrosplenic trunk with HM trunk |
|
6. |
GSp + CHA + SMA |
Gastrosplenic trunk with CHA and SMA arising from the aorta |
Abbreviations: CHA, common hepatic artery; CM, celiomesenteric HM, hepatomesenteric; LGA, left gastric artery; SMA, superior mesenteric artery.
We analyzed patterns of aortic origin for the four major arteries, the LGA, CHA, SpA and SMA, with adherence to the modified definition of celiac axis (as discussed above) and by defining normal CHA6 as an arterial trunk giving rise to right hepatic artery (RHA) or left hepatic artery (LHA) and the GDA, irrespective of its origin and course. For patients in whom a variant was identified, the specific type of variant was recorded according to Michel’s criteria7 (Table 2).
|
Types |
Description |
|---|---|
|
I |
Hepatic artery originates from the CHA and bifurcates into the RHA and LHA |
|
II |
Replaced LHA arising from LGA |
|
III |
Replaced RHA arising from SMA |
|
IV |
Replaced RHA and LHA |
|
V |
Accessory LHA arising from LGA |
|
VI |
Accessory RHA arising from SMA |
|
VII |
Accessory RHA arising from SMA and accessory LHA arising from LGA |
|
VIII |
Replaced RHA and accessory LHA Replaced LHA and accessory RHA |
|
IX |
The CHA arising from SMA |
|
X |
The CHA arising from LGA |
|
XI |
For any variant not described for types I–X |
Abbreviations: CHA, common hepatic artery; LGA, left gastric artery; LHA, left hepatic artery; SMA, superior mesenteric artery; RHA, right hepatic artery.
aReplaced: replaced origin of hepatic arteries refers to the arterial blood supply from an ectopic location.
bAccessory: accessory origin of hepatic arteries refers to the arterial blood supply from typical as well as ectopic branch.
Results
Following are the results in the effective sample of 124 patients (four patients were excluded from the study due to motion artifact.)
-
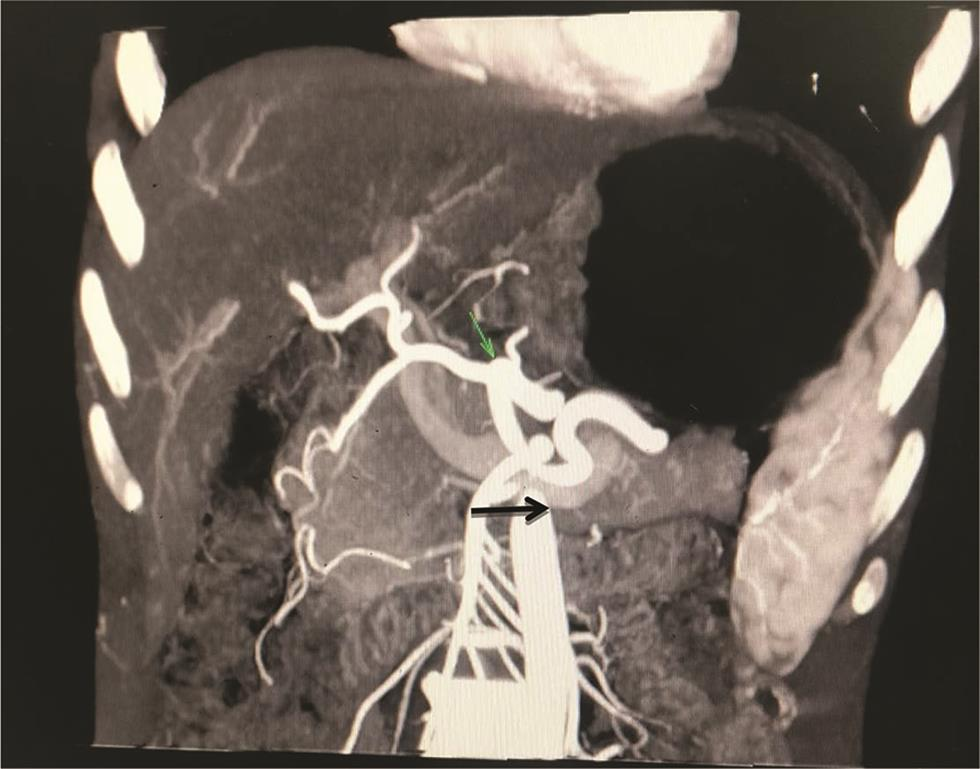
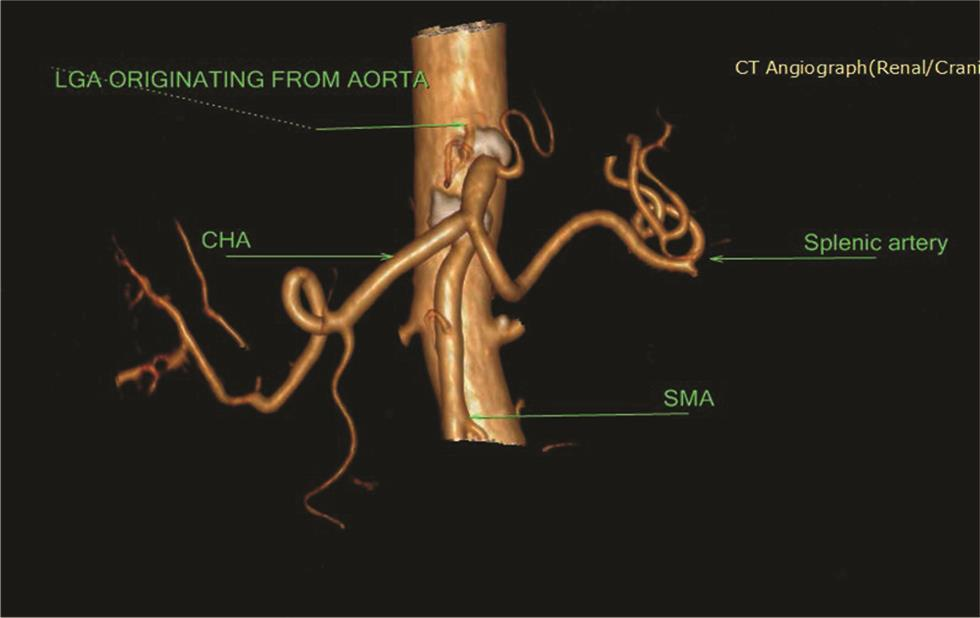
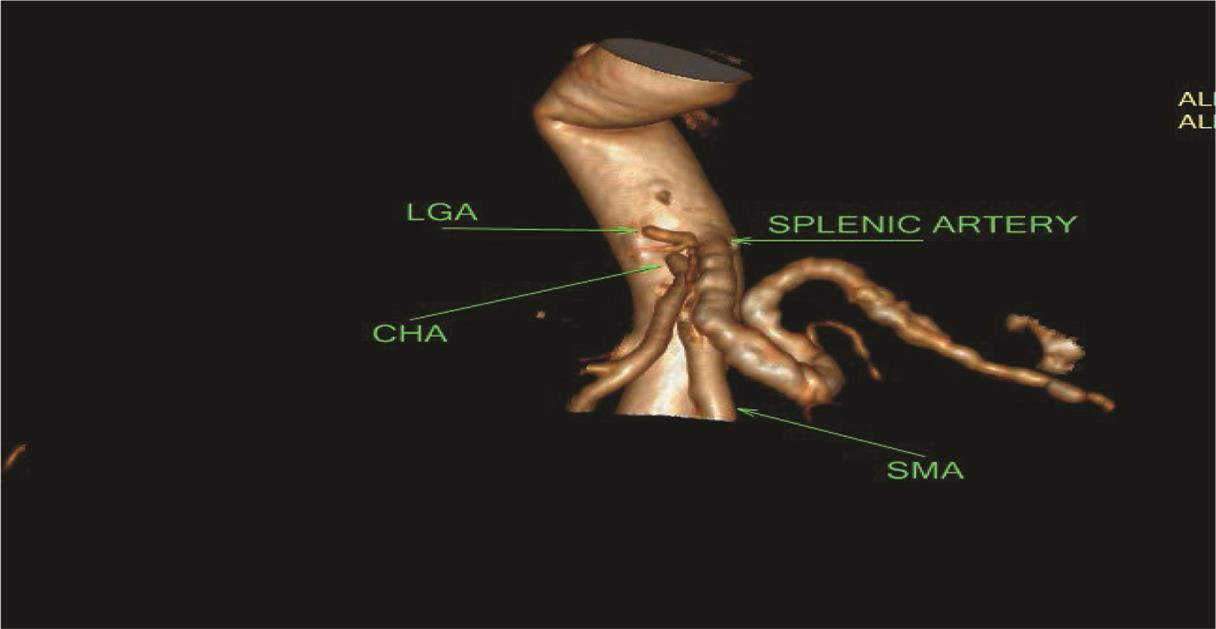
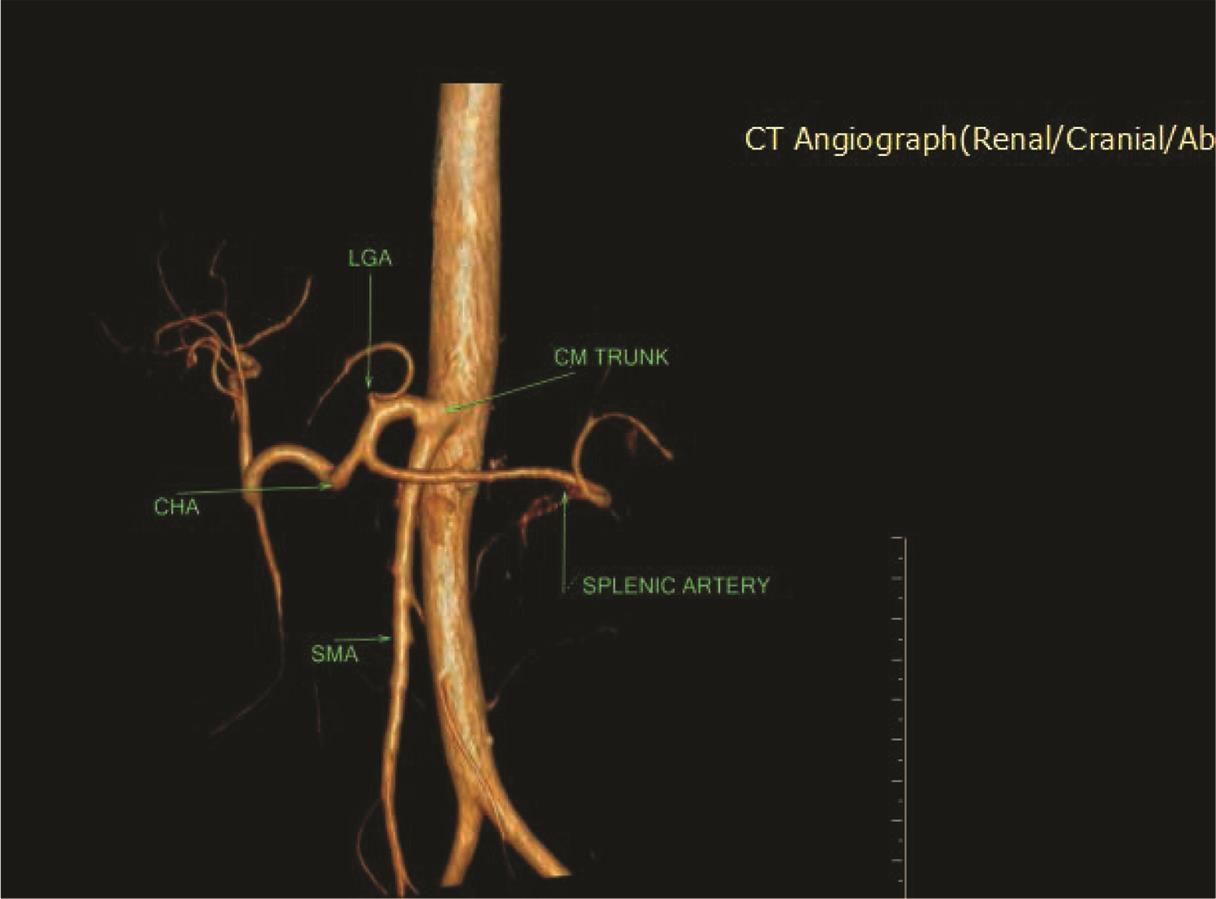
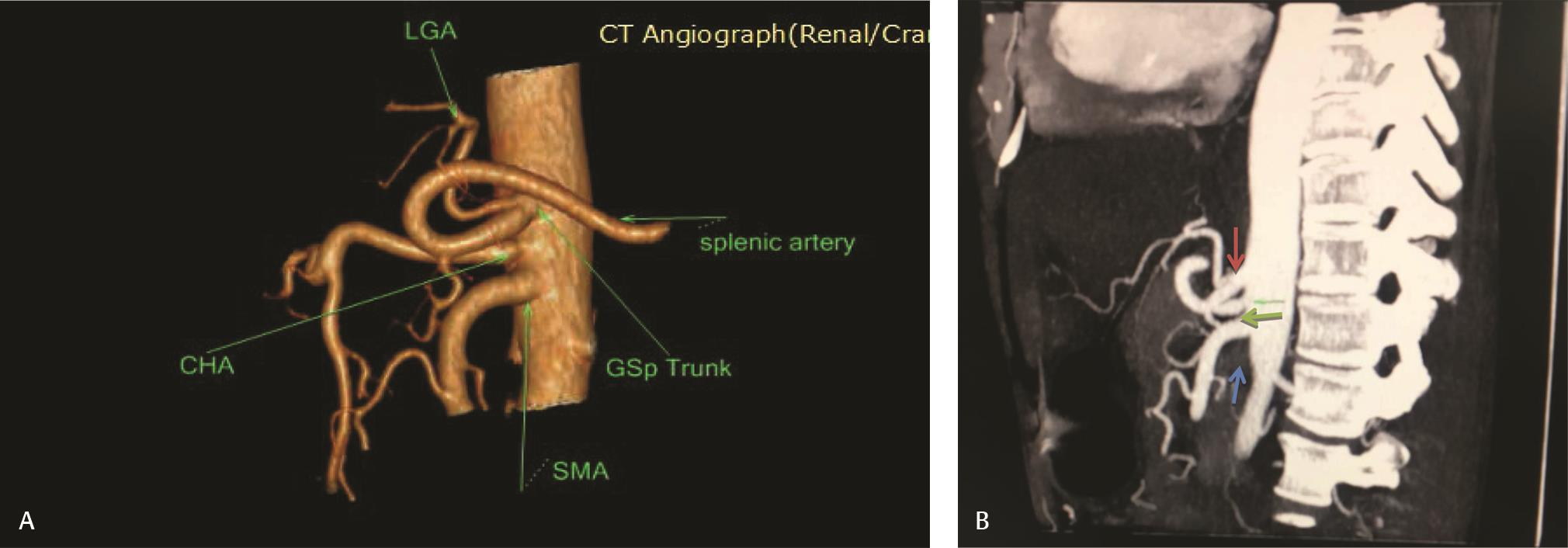
Five types of celiac axis and SMA anatomic variations was found in the effective study sample of 124 patients (Table 3; Fig. 1). Maximum number of cases (n = 115; 92.7%) showed classical celiac axis (Fig. 2) and SMA branching pattern findings. There were four (3.2%) cases showing LGA + Hsp + SMA (Fig. 3) and two (1.6%) showed GSp + HM branching patterns (Fig. 4). There was one (0.8%) case each showing LGA + CHA + SpA + SMA (Fig. 5), CM trunk (Fig. 6), and GSp + HA + SMA (Fig. 7AB) patterns.
-
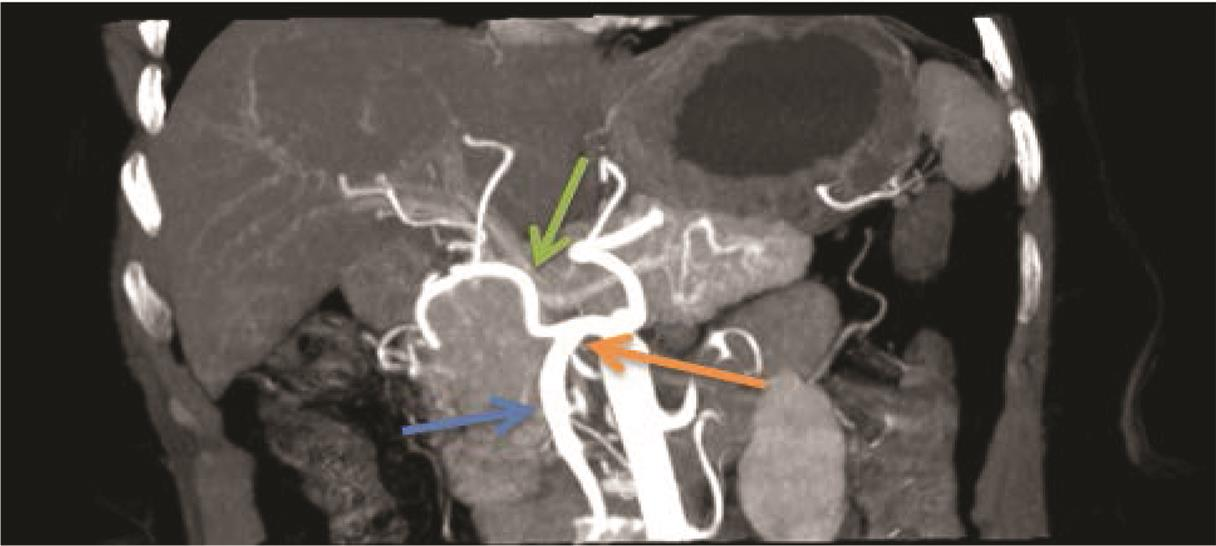
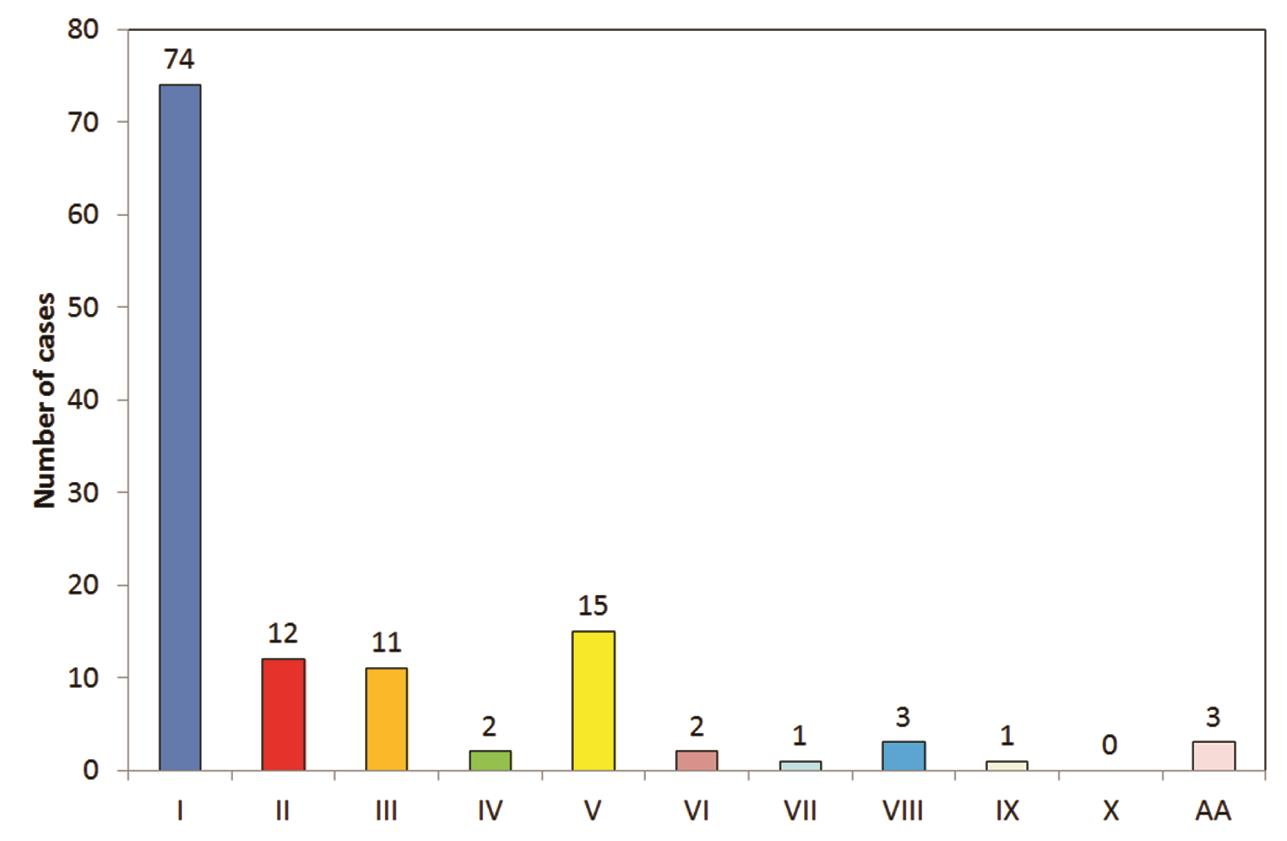
The findings for variation in hepatic artery branching are shown in Table 4Fig. 8. Majority of cases showed pattern I (n = 74; 59.6%; Fig. 9) followed by patterns V (n = 15; 12.1%; Fig. 10), II (n = 12; 9.7%; Fig. 11AB), and III (n = 11; 8.9%; Fig. 12AB). There were three (2.4%) cases each showing pattern VIII (Fig. 13) and AA and two (1.6%) cases each showing patterns IV (Fig. 14) and VI (Fig. 15), respectively. There was one (0.8%) case each showing pattern VII and IX (Fig. 16; Table 3). A total of three (2.4%) cases showed RHA arising from celiac axis.
|
S. No. |
Finding |
No. of cases |
Percentage |
|---|---|---|---|
|
1. |
Classical |
115 |
92.7 |
|
2. |
LGA + HSp + SMA |
4 |
3.2 |
|
3. |
LGA + CHA + SpA+ SMA |
1 |
0.8 |
|
4. |
CM Trunk |
1 |
0.8 |
|
5. |
GSp + HM |
2 |
1.6 |
|
6. |
GSp +HA + SMA |
1 |
0.8 |
Abbreviations: CHA, common hepatic artery; CM, celiomesenteric; GSp, gastrosplenic; HA, hepatic artery; HM, hepatomesenteric; HSp, hepatosplenic; LGA, left gastric artery; SMA, superior mesenteric artery.
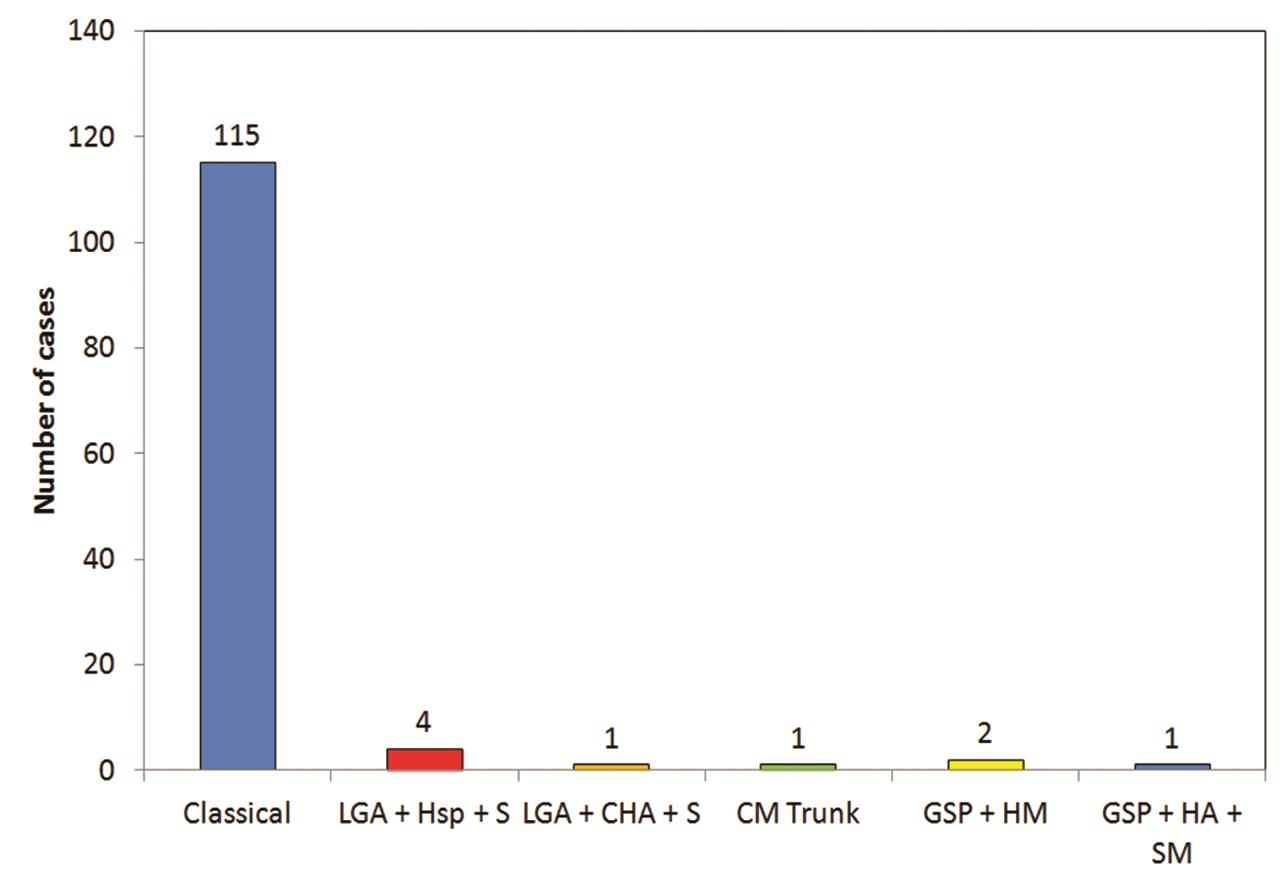
-
Fig. 1 Distribution of cases according to celiac axis and SMA findings. SMA, superior mesenteric artery.
-
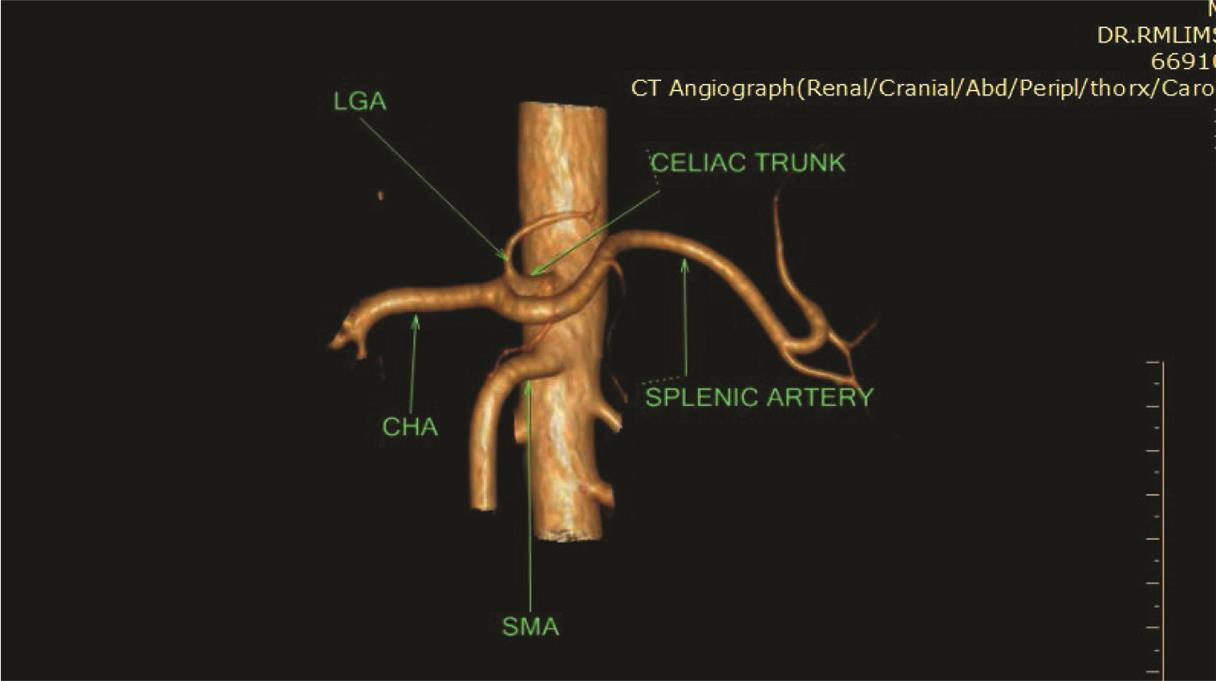
Fig. 2 Coronal maximum intensity projection image showing classical celiac axis (green arrow) and splenic artery (purple), LGA (yellow), CHA (orange) originating from CA, and SMA (black arrow) branching pattern. 3D, three-dimensional; CTA, computed tomography angiography; VR, volume rendering.
-
Fig. 3 3D VR CTA LGA originating in the aorta, immediately above the trunk. The splenic artery nd common hepatic artery originate from the same trunk. 3D, three-dimensional; CTA, computed tomography angiography; LGA, left gastric artery.
-
Fig. 4 Maximum intensity projection image on coronal plane showing hepatomesenteric trunk (orange arrow), SMA (blue arrow), CHA (green arrow). CHA, common hepatic artery; SMA, superior mesenteric artery.
-
Fig. 5 3D VR CTA the CHA, LGA, SA and SMA arising separately from the aorta; this rare variation is known as the absence of the CA. 3D, three-dimensional; CHA, common hepatic artery; CTA, computed tomography angiography; LGA, left gastric artery; SA, splenic artery; SMA, superior mesenteric artery; VR, volume rendering.
-
Fig. 6 3D VR CTA showing CMT. 3D, three-dimensional; CTA, computed tomography angiography; VR, volume rendering.
-
Fig. 7 (A) 3D VR CTA shows separate origin of CHA, GSp trunk and SMA. (B) Sagittal MIP image showing separate origin of CHA (green arrow). GSp trunk (red arrow) is arising just above and SMA (blue) is arising just below it. 3D, three-dimensional; CHA, common hepatic artery; CTA, computed tomography angiography; GSp, gastrosplenic; MIP, maximum intensity projection image; SMA, superior mesenteric artery ; VR, volume rendering.
|
S. No. |
Findings |
No. of cases |
Percentage |
|---|---|---|---|
|
1. |
I |
74 |
59.5 |
|
2. |
II |
12 |
9.7 |
|
3. |
III |
11 |
8.9 |
|
4. |
IV |
2 |
1.6 |
|
5. |
V |
15 |
12.1 |
|
6. |
VI |
2 |
1.6 |
|
7. |
VII |
1 |
0.8 |
|
8. |
VIII |
3 |
2.4 |
|
9. |
IX |
1 |
0.8 |
|
10. |
X |
0 |
0 |
|
11. |
AA |
3 |
2.4 |
-
Fig. 8 Findings according to Michel’s classification. 3D, three-dimensional; CTA, computed tomography angiography.
-
Fig. 9 VR 3D CTA showing Michel’s type-I variant. 3D, three-dimensional; CTA, computed tomography angiography; VR, volume rendering.
-
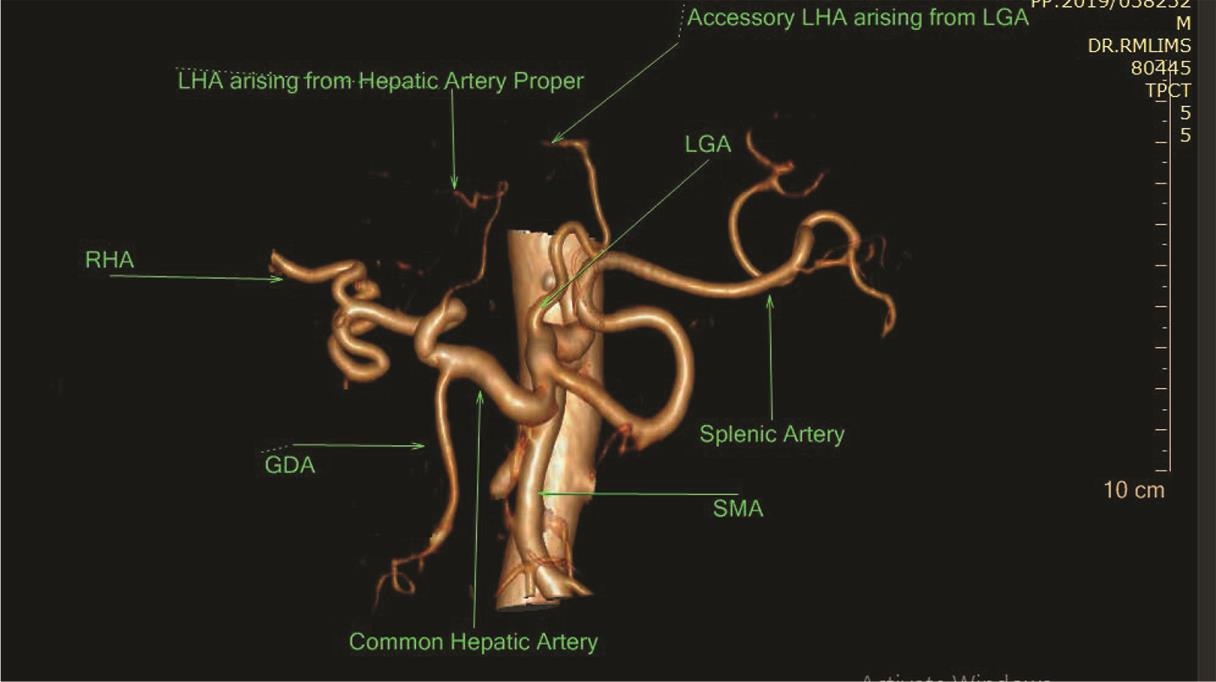
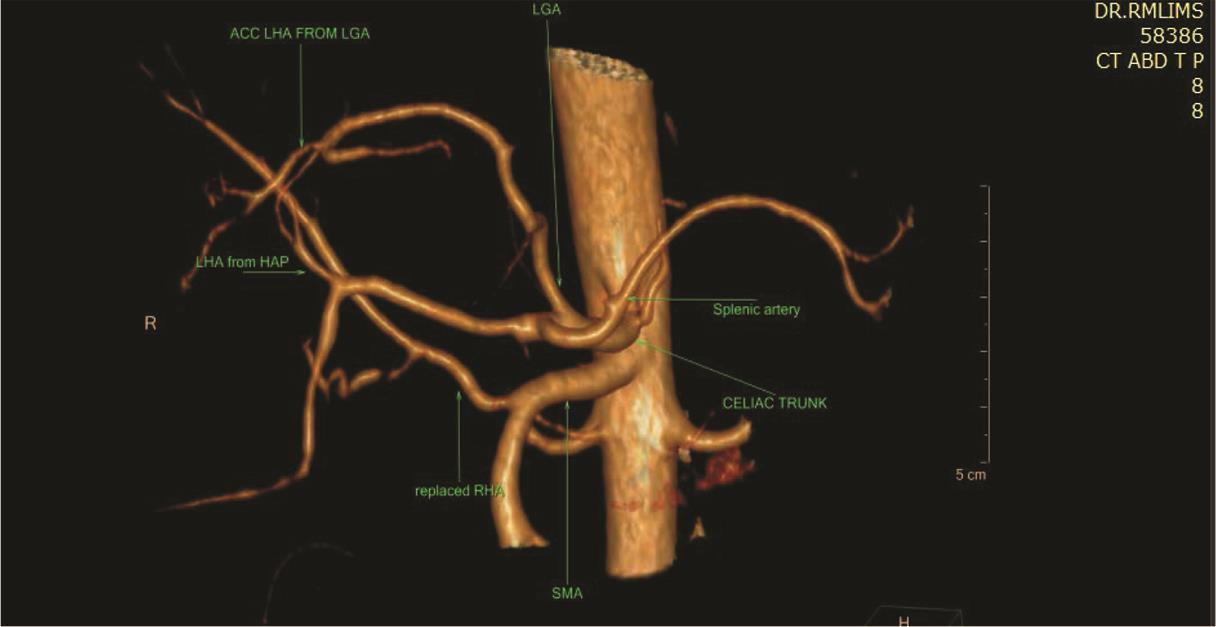
Fig. 10 3D VR CTA showing Michel’s classification type V hepatic artery anatomy. Acc. LHA arising from LGA. 3D, three-dimensional; CTA, computed tomography angiography; LHA, left hepatic artery; LGA, left gastric artery; VR, volume rendering.
-
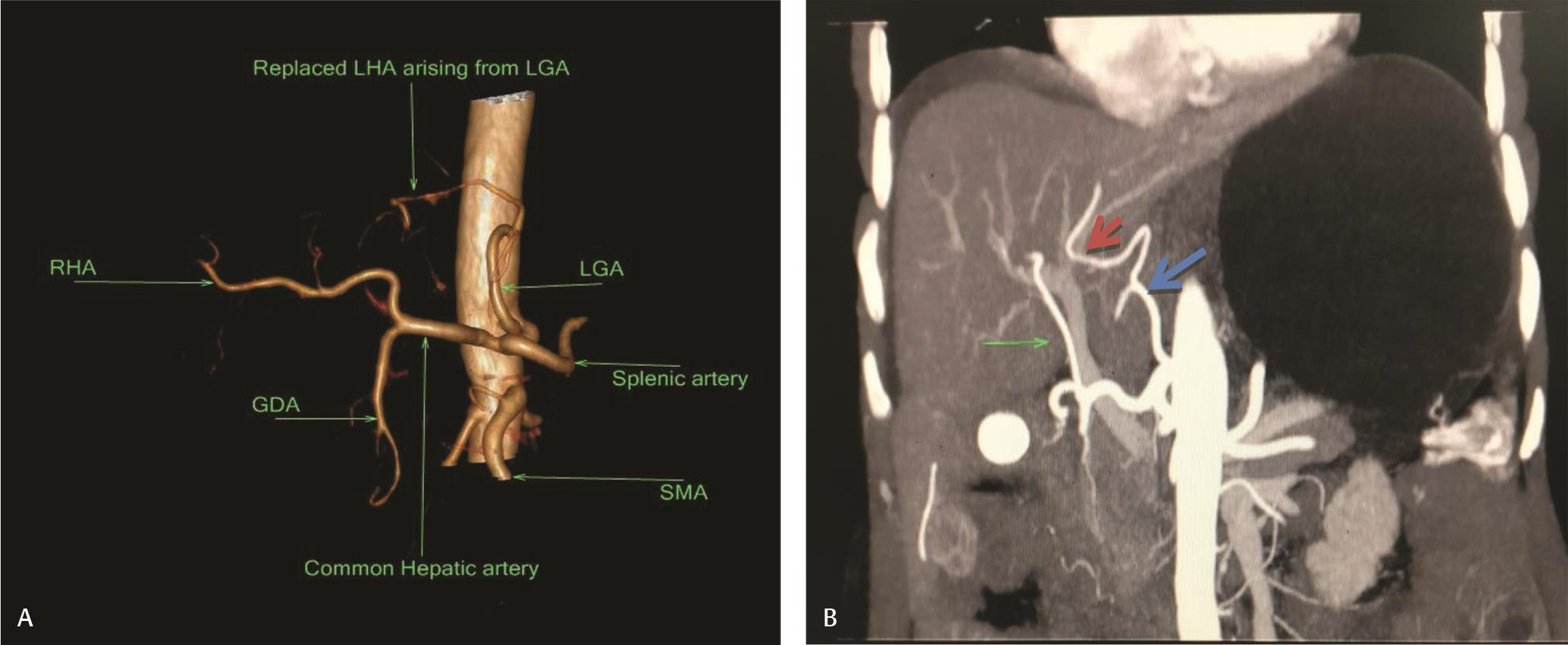
Fig. 11 (A) Type II variant showing replaced LHA arising from LGA. (B) Coronal MIP Image showing Replaced LHA arising from (red arrow) arising from left gastric artery (blue arrow). Middle hepatic artery (large arrow) arising from hepatic artery proper. LGA, left gastric artery; LHA, left hepatic artery; MIP, maximum intensity projection image.
-
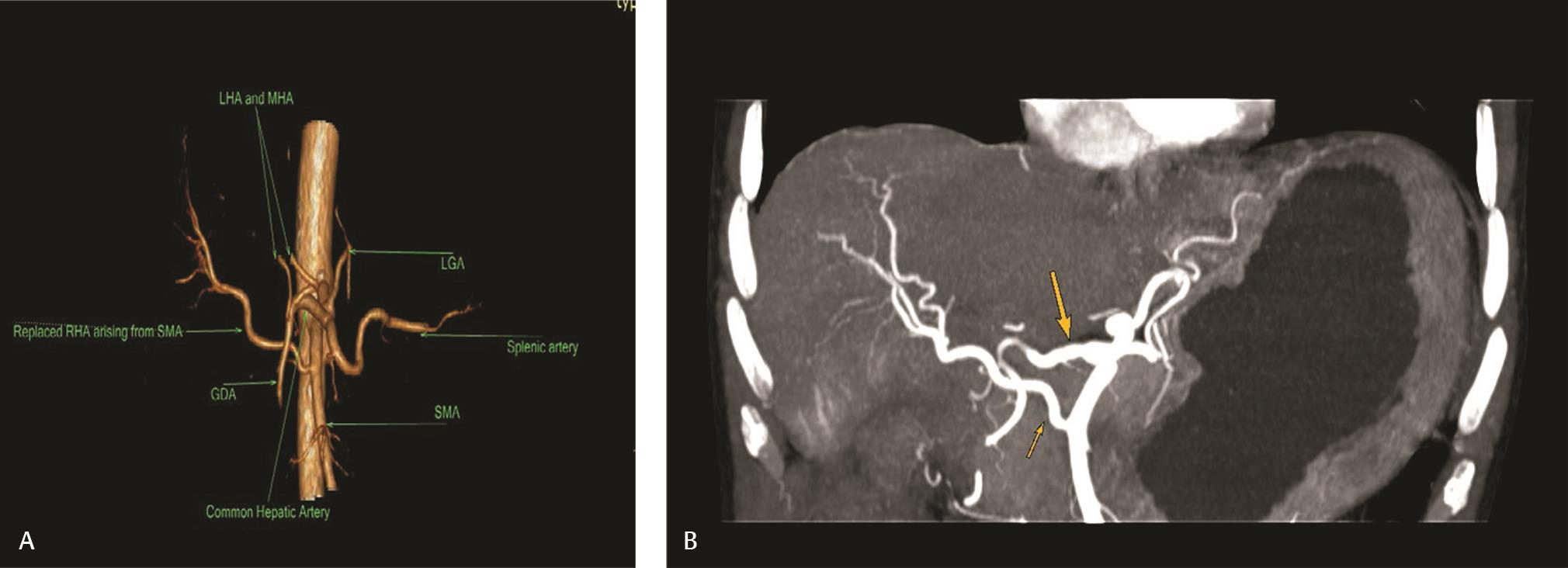
Fig.12 (A) Type III variant showing replaced RHA arising from SMA. (B) MIP on coronal plane showing type III hepatic artery anatomy. Replaced RHA (small arrow) arising from SMA and CHA (large arrow) is continuing as GDA. CHA, common hepatic artery; GDA, gastroduodenal artery MIP, maximum intensity projection image; RHA, right hepatic artery; SMA, superior mesenteric artery.
-
Fig. 13 VR 3D CTA showing Michel’s classification type VIII. Rpl. RHA arising from SMA and Acc. LHA arising from LGA. 3D, three-dimensional; CTA, computed tomography angiography; LGA, left gastric artery; RHA, right hepatic artery; SMA, superior mesenteric artery; VR, volume rendering.
-
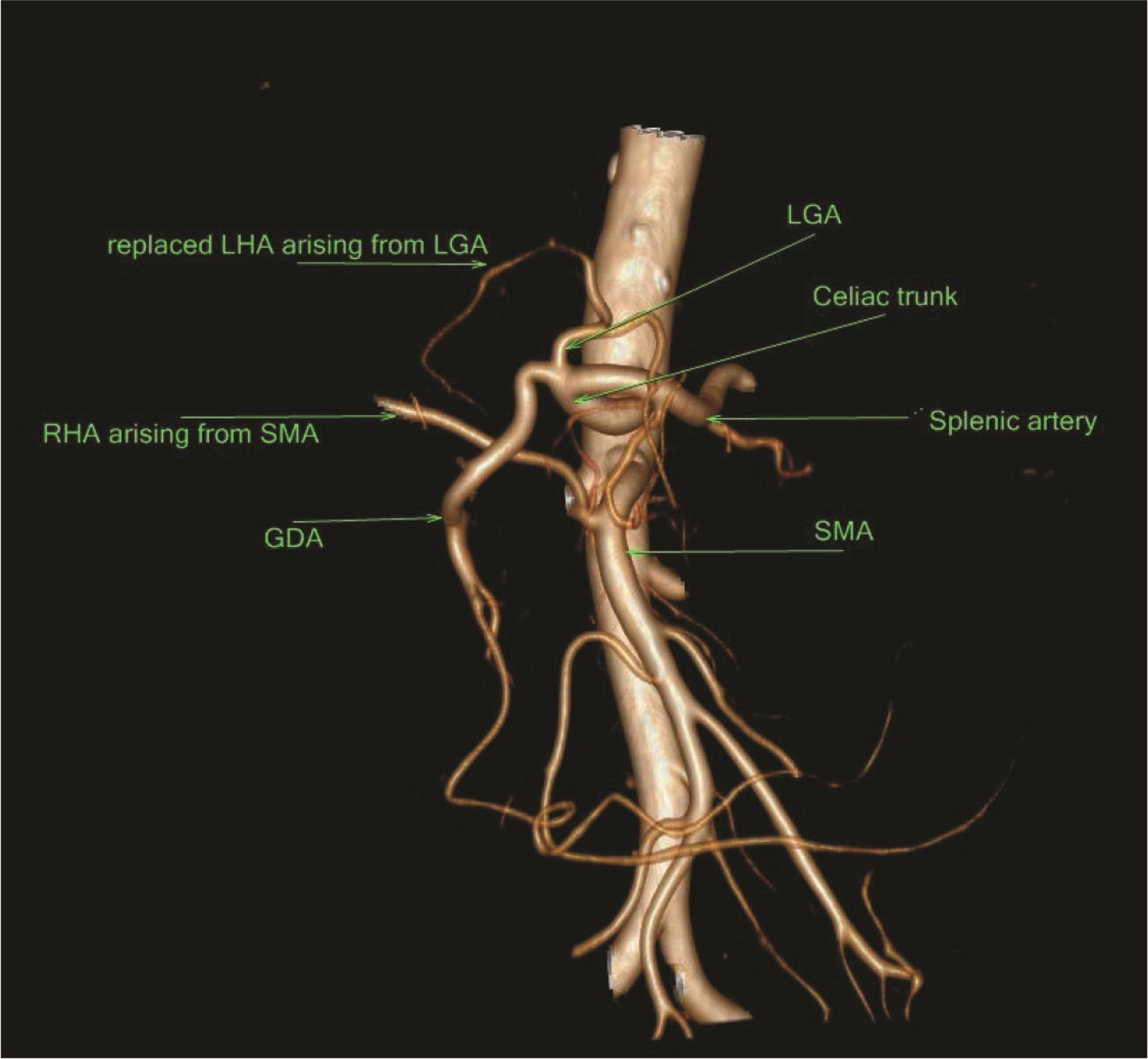
Fig. 14 Type IV showing both replaced LHA and RHA. LHA, left hepatic artery; RHA, right hepatic artery.
-
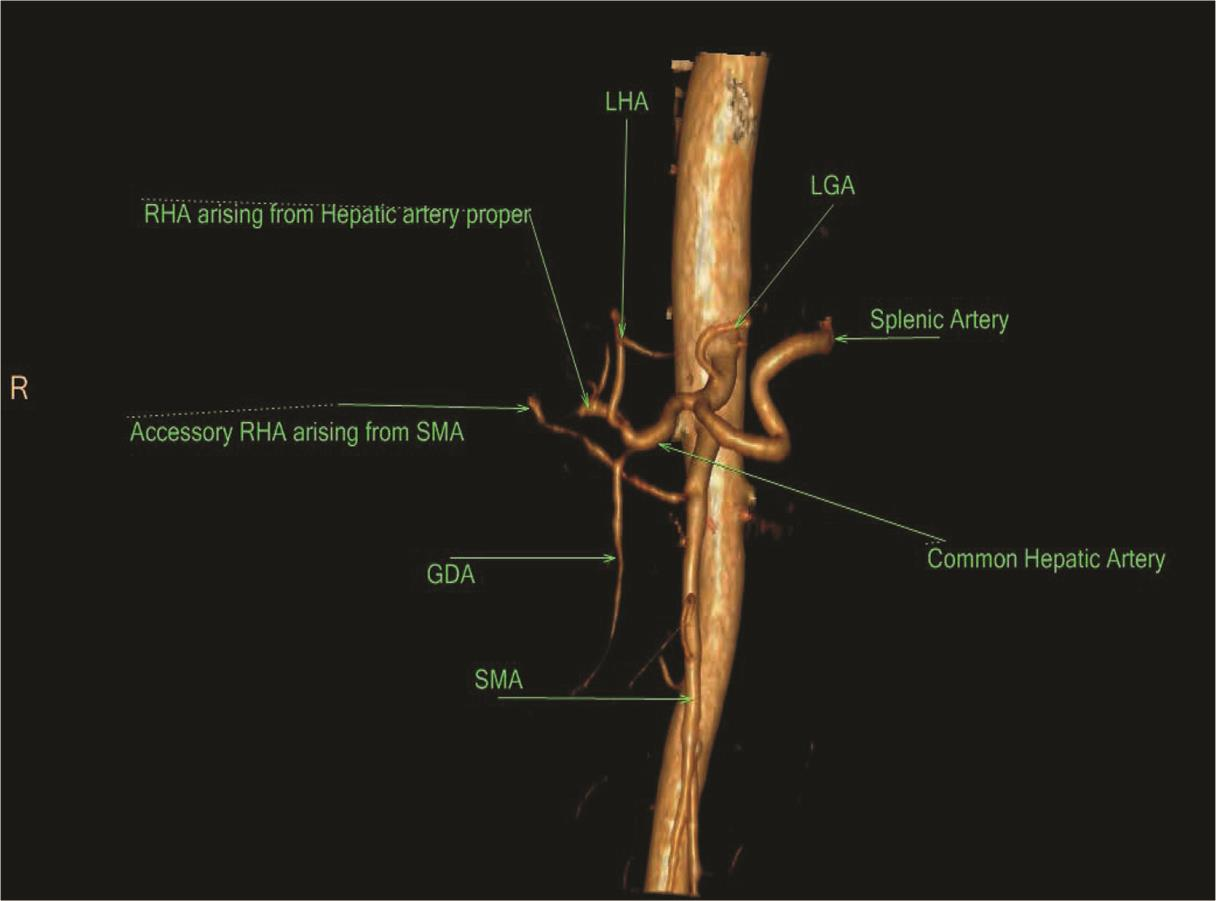
Fig. 15 3D VR CTA showing type VI variant showing accessory RHA arising from SMA. 3D, three-dimensional; CTA, computed tomography angiography; RHA, right hepatic artery; SMA, superior mesenteric artery; VR, volume rendering.
-
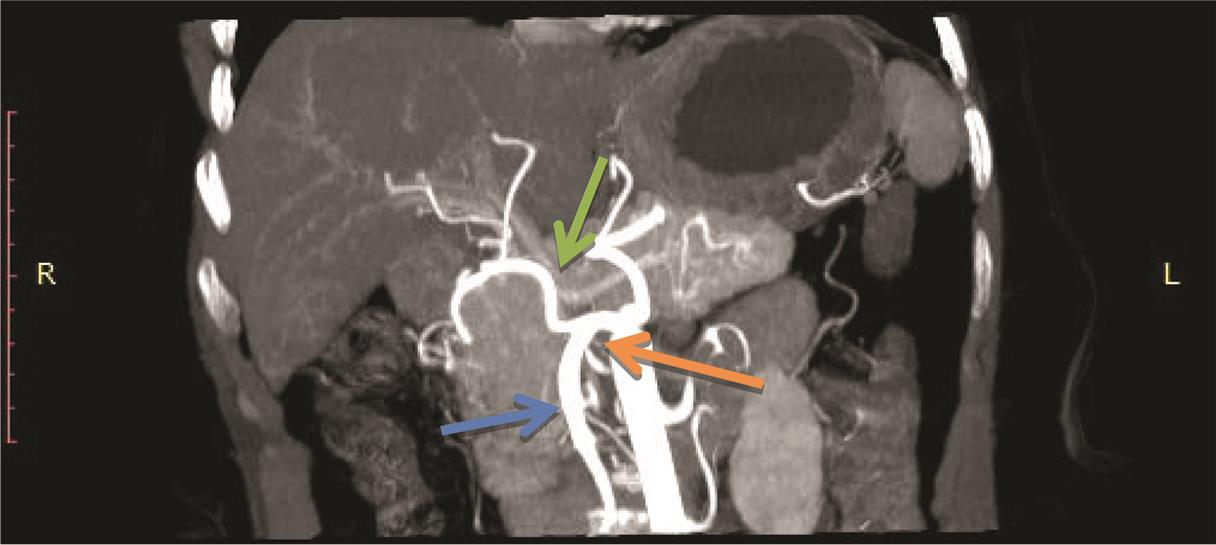
Fig. 16 Maximum intensity projection image on coronal plane showing Michel’s classification type IX. Hepatomesenteric trunk (orange arrow), SMA (blue arrow), CHA (green arrow). CHA, common hepatic artery; SMA, superior mesenteric artery.
Discussion
Celiac Axis Variation
In our study, maximum number of cases (n = 115; 92.7%) showed classical celiac axis anatomy which is similar to the study of Michel,7 Song et al,6 Thangarajah and Parthasarathy,8 and Wang et al5 in which the prevalence of a normal celiac axis anatomy is 89, 89.1, 89.5, and 89.8%, respectively.
Five of the possible 15 types of variants in 8 (7.2%) of the patients (Table 2Fig. 2) were identified in our study. This was in contrast to 12 types of celiac axis variations in 9.64% of patients in the study of Song et al,6 seven types observed in 10.2% of the patients in the study of Wang et al5 and six types of celiac axis variations in 5.5% of cases in the study of Sureka et al.9
The most common anatomic variation was separate origin of each HSp, LGA, and SMA from aorta found in (3.2%) patients. This result was similar with the results obtained by Song et al (4.42%).6 However, study of Sureka et al9 and Wang et al5 showed the same in 2.83 and 0.27% cases, respectively.
The next common anatomic variation in our study was that of gastrosplenic trunk with hepatomesenteric trunk (1.6%). Sureka et al9 observed the same in 0.66%.This was the most common variant in the study of Wang et al5 and was detected in 4.13% of the cases.
In our study, one (0.8%) case showed common celiomesenteric trunk. The same was observed in 0.66% case in the study of Sureka et al9 and in 3.1% cases in the study of Dao et al.10
In our study, one case (0.8%) showed the separate origin of LGA, CHA, SpA, and SMA(namely, absence of the celiac trunk) as compared with 0.6% cases in the study of Iezzi et al,11 0.2% cases in the study of Wang et al,5 and 0.24% cases in the study of Gümüs et al.12
One case (0.8%) showed separate branching of gastro splenic trunk, hepatic artery, and SMA branching. This was close to the results achieved by Sureka et al (0.33%)9 and Awad et al (1.3%).13
In our study, normal CHA anatomy in which the CHA originated from the celiac axis was seen in 122 (98.38%) of the cases, while in the study of Sureka et al9 and Thangarajah and Parthasarathy,8 normal CHA origin was seen in 95.83 and 94% of the cases, respectively.
A separate origin of the CHA from the aorta is a rare variation, and the prevalence of this variation in our study was only 1.6% (two cases) which is comparable to 2.8% cases in the study of Awad et al.13
Thus the prevalence of variations of celiac axis is very variable according to the populations surveyed. Such a difference could depend on genetic characteristics, the exact definition of the criteria for analysis, and degree of resolution of the images.
Common Hepatic Artery Variations
Our study type I or the normal branching pattern was found in 74 cases (59.5%) which was similar with the findings of Covey et al (61.3%)14 and Gümüs et al (66%).12
Variations in the origin of the hepatic arteries was noted in 50 (40.3%) patients. In our study, 40 (32.3%) patients had a single arterial variant identified, and 6 (4.8%) patients had more than one arterial variant; three (2.4%) cases among which had a combination of accessory and replaced hepatic arteries. This was different than the study of Dao et al10 who observed 20% of cases had one anatomical variant and 9.4% had two anatomical variants.
The most common variant in our study was type V (12.1%), followed by types II (9.7%) and type III (8.9%). Löschner et al15 and Thangarajah and Parthasarathy8 also reported type V as the most common variant in their study contributing to 8.8 and 8.5% cases, respectively. In the study of Egorov et al,16 the most common variant was type III (14%), followed by types V (8%) and II (4.6%). Gümüs et al12 observed the most common abnormality which was Michel’s type III (10.1%), followed by types V (7.3%) and II (4.7%). In the study of De Cecco et al,17 the most common anomaly was Michel’s type III (9.2%), followed by types II and V (5.2%).
LHA origin from CHA was seen in 91 (81.5%) patients.The most common variant found was accessory LHA arising from LGA found in 16 (12.9%) patients which was similar with the study of Covey et al (11%)14 and higher than study of Sureka et al (7.6%) cases,9 Winston et al (4%),18 and of Farghadani et al (1.5%).19 Replaced origin of LHA from the LGA was seen in 11.3% (14 cases), 6.6% cases were found in the study of Farghadani et al,1910.6% in the cases of Sureka et al,9 and 8% cases in Winston et al.18
RHA origin from CHA was seen in 81.6% (102) of the cases which was similar with the study of Sureka et al (79.6%).9 The most frequent variation of RHA was replaced origin of RHA from SMA seen in 10.4%13 of the cases. It was found in 9.6% cases in the study of Farghadani et al,19 13% cases in study of Sureka et al,9 15% of patients in the study of Winston et al,18 and 12.6% in the study of Awad et al.13 The other site of origin of replaced RHA was from celiac axis (ambiguous anatomy) seen in three cases (2.4%) as compared with 1.8% in the study of Farghadani et al19 and 1.4% in study of Awad et al.13 Accessory origin of RHA was seen in three (2.4%) of the cases as compared with the 0.5% cases of Farghadani et al,19 5.16% cases of Sureka et al,9 and 1% cases of Winston et al.18
In our study type II was found in 9.7%, which was similar to the study of López-Andújar et al (9.7%).20 In our study, type III was found in 8.9% as compared with 8.7% in the study of Covey et al14 and 10.1% in the study of Gümüs et al.12
In our study, type IV was found in 1.6% of the cases, nearly similar to as compared with 2.8% cases in the study of Awad et al13 and 0.7% cases in the study of Gümüs et al.12
In our study, type VI was found in 1.6%2 cases which was similar to the study of Covey et al (1.5%)14. Same variation was seen in 3.4% cases in the study of Gümüs et al.12
In our study, type VII was found in 0.8%1 case which was close to the observation of Gümüs et al (1.21%)12 and Covey et al (1%).14
In our study, type VIII was found in 2.4%3 cases similar to the findings of Covey et al (3%)14 and Gümüs et al (2.3%).12
In our study, type IX was found in 0.8%1 case which was similar to the observations of Gruttadauria et al21 (0.86%) cases. Farghadani et al19 noted the same in 1.3% of the cases. Awad et al13 noted the same in 4.2% of the cases. Type X was not found in our study.
Results
After evaluation of hepatic artery, majority of cases showed pattern I (59.6%), followed by patterns V (12.1%), II (9.7%), and III (8.9%). There were three (2.4%) cases each showing patterns VIII and AA and two (1.6%) cases each showing patterns IV and VI, respectively. There was one (0.8%) case each showing pattern VII and IX. A total of three (2.4%) cases showed RHA arising from celiac axis.
Conclusion
We conclude that most common pattern of celiac axis and SMA branching is classical pattern (92.7%) which is in concordance with literature.
Type-I pattern of hepatic artery branching was most common (59.6%), similar to that documented in literature. Although the most common variation in our study is type V (12.1%), followed by types II (9.7%) and III (8.9%), whereas the most common variation in many literature was found to be type III. CT angiography hence is an excellent diagnostic modality for depiction of arterial anatomic variations and provides a roadmap for surgical treatment.
Conflict of Interest
None declared.
References
- Hepatic arterial anatomy: demonstration of normal supply and vascular variants with three-dimensional CT angiography. Radiographics. 1995;15(04):771-780.
- [Google Scholar]
- The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR Am J Roentgenol. 1997;168(04):971-977.
- [Google Scholar]
- Impact of double helical CT and three-dimensional CT arteriography on surgical planning for hepatic transplantation. Abdom Imaging. 1999;24(03):278-284.
- [Google Scholar]
- Hepatic arteries in potential donors for living related liver transplantation: evaluation with multi-detector row CT angiography. Radiology. 2003;227(02):391-399.
- [Google Scholar]
- Identification of spectrum and prevalance of anatomical variations in the origin of CA, SMA and their major branches by MDCT Angiography. Eur Radiol. 2014;24:1777-1784.
- [Google Scholar]
- Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010;255(01):278-288.
- [Google Scholar]
- Blood supply and anatomy of the upper abdominal organs, with a descriptive atlas Philadelphia, PA: Lippincott; 1955. p. :64-69.
- [Google Scholar]
- Celiac axis, common hepatic and hepatic artery variants as evidenced on MDCT angiography in South Indian population. J Clin Diagn Res. 2016;10(01):TC01-TC05.
- [Google Scholar]
- Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging. 2013;23(03):223-233.
- [Google Scholar]
- Anatomical variants of celiac trunk in relation to its branching: a preliminary sub-saharan study. Open Journal of Radiology. 2019;9:151-161.
- [Google Scholar]
- Multidetector-row CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat. 2008;30(04):303-310.
- [Google Scholar]
- Variations of the celiac trunk and hepatic arteries: a study with 64-detector computed tomographic angiography. Eur Rev Med Pharmacol Sci. 2013;17(12):1636-1641.
- [Google Scholar]
- Anatomical variations of celiac trunk: an angiographic study. Med J Cairo Univ. 2017;85:119-126.
- [Google Scholar]
- Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224(02):542-547.
- [Google Scholar]
- Hepatic arterial supply in 1297 CT-angiographies. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2015;187(04):276-282.
- [Google Scholar]
- Celiaco-mesenterial arterial aberrations in patients undergoing extended pancreatic resections: correlation of CT angiography with findings at surgery. JOP. 2010;11(04):348-357.
- [Google Scholar]
- Anatomic variations of the hepatic arteries in 250 patients studied with 64-row CT angiography. Eur Radiol. 2009;19(11):2765-2770.
- [Google Scholar]
- CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol. 2007;189(01):W13-9.
- [Google Scholar]
- Anatomical variation of celiac axis, superior mesenteric artery, and hepatic artery: Evaluation with multidetector computed tomography angiography. J Res Med Sci. 2016;21:129.
- [Google Scholar]
- Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver Transpl. 2007;13(10):1401-1404.
- [Google Scholar]
- The hepatic artery in liver transplantation and surgery: vascular anomalies in 701 cases. Clin Transplant. 2001;15(05):359-363.
- [Google Scholar]







