Translate this page into:
An audit of gastrointestinal stromal tumors from a tertiary medical college hospital in Eastern India
Address for correspondence: Dr. Samrat Dutta, Department of Radiotherapy, North Bengal Medical College, Sushrutanagar, Siliguri - 734 012, West Bengal, India. drsamratdutta@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction: Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of GI tract. They are recently recognized as a distinct pathological entity although previously they were grouped with sarcomas of smooth muscle origin, i.e., leiomyomas, leiomyoblastomas, or leiomyosarcomas. However, apart from GI tract, GIST can occur in any smooth muscle-like in the urinary bladder. Proper diagnosis by immunohistochemistry stain CD133 and risk stratification by morphological parameters has been the cornerstone of treatment.
Materials and Methods: This study was conducted as a retrospective analysis to audit the number of cases presenting as GIST in the tertiary medical colleges of eastern India and find out the patterns of care with the available modalities of therapy.
Results: Out of total 15 cases, the median age of presentation was 45 years; the Male: Female (M: F) ratio was 2:3 and persisting dragging prolonged chronic abdominal pain was present in the majority. Intestinal complications were few (20%). All were treated with imatinib mesylate 400 mg once daily. However, two patients progressed for whom the dose of imatinib was escalated and one patient was metastatic at onset who was later switched over to sorafenib even after disease progression with dose escalation with imatinib. The median follow-up was 17.5 months and the median time to response to imatinib was 3.2 months. The 2-year actuarial overall survival was 79.12%, and progression-free survival was 86.67%.
Conclusions: The future directions are to determine appropriate duration of imatinib therapy in adjuvant/neoadjuvant and therapeutic setting.
Keywords
Gastrointestinal stromal tumors
imatinib mesylate
prognosis
Introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the GI tract. They were previously grouped with sarcomas of smooth muscle origin, i.e., leiomyomas, leiomyoblastomas, or leiomyosarcomas. Recently, they are recognized as a distinct pathological entity. They represent 0.52% of all GI malignancies.1 The incidence is 20,000 yearly worldwide and the prevalence is 10–20/1,000,000.2 The most common site of initial presentation is stomach - 60%–70% and small intestine - 20%–25%. Although rarely they may occur in colon, rectum, esophagus, and urinary bladder.3
The expression of c-kit (CD117) tumor marker is seen in nearly 95% cases.4 Although other tumor markers such as CD34, smooth muscle antigen, s-100, and desmin are also observed.5 The receptor tyrosine kinase is a protein product of the c-kit antigen which is detected by immunohistochemistry (IHC) as CD117 antigen. The receptor has an extracellular part to bind with the growth factor stem cell factor (SCF) and an intracellular part which has tyrosine kinase activity Figure 1. When there is SCF ligand binding it results in abnormal continuous c-kit signaling involved in the development of GIST.6
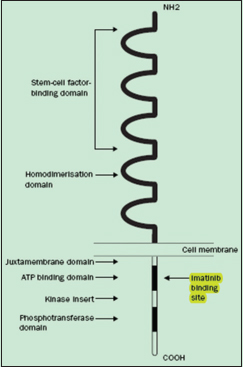
- Molecular structure of c-kit receptor
Imatinib mesylate (formerly called ST-571) targets and inhibits c-kit activation and hence is used in adjuvant and neoadjuvant therapy of GIST.
This study was conducted in two tertiary care medical college hospitals in the state of West Bengal to understand the incidence of GIST malignancy and also to evaluate the efficacy of imatinib as its role in adjuvant and therapeutic setting. The two hospitals cater to a huge population of cancer patients owing to their locations – (1) Kolkata - the capital of West Bengal and (2) Siliguri - the important place in North Bengal (also known as the capital of north Bengal) which receives referrals from 6 districts of north Bengal, 3 adjoining states – Sikkim, Bihar, and Assam, and also from 3 adjoining neighboring countries – Nepal, Bhutan, and Bangladesh. Hence, this study will also serve to understand the incidence, prevalence, and the treatment outcomes with imatinib in patients suffering from GIST in the eastern part of the country.
Aims and objectives
This study aims to understand the patterns of presentation and disease response to first-line imatinib mesylate therapy.
Materials and Methods
Inclusion criteria
-
All patients having biopsy proven suggestive of stromal malignancy and IHC positive for CD117 Figures 23
-
No prior treatment received.
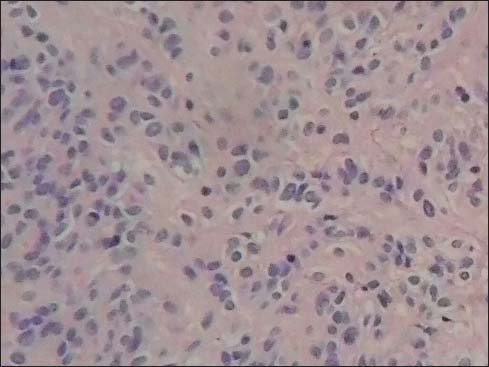
- Light microscopy high power view of the histology showing spindle cells of the gastric stromal tumor
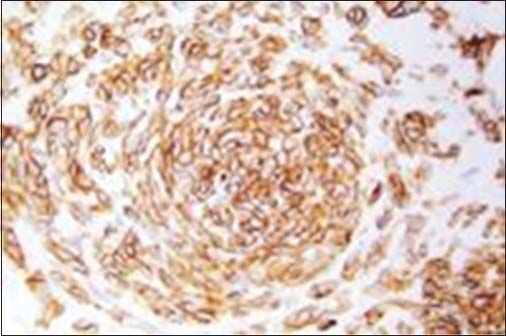
- Immunohistochemistry showing CD117 positivity
Exclusion criteria
-
Uncertain biopsy report and IHC negative for CD117
-
Metachronous and/or synchronous malignancy
-
No prior comorbid illness such as diabetes mellitus, tuberculosis, coronary artery disease, autoimmune disorders, allergy, and dermatitis.
All patients who attended the radiotherapy departments at North Bengal Medical College, Siliguri and of Institute of Postgraduate Education and Research (IPGMER), Kolkata, fulfilling all the inclusion and exclusion criteria were studied as per departmental records maintained in the respective files.
All patients had initial workup with blood parameters including complete blood counts, liver function tests, renal function tests, electrolytes, echocardiography, chest X-ray, ultrasonography (USG), and/or computed tomograms (CT) scans of the whole abdomen. Treatment response was evaluated at 1 month of therapy by USG and/or CT scan and then at 4–6 monthly interval thereafter using the RECIST criteria.
The study was done from April 2016 to January 2018. Out of the total, 15 patients registered as GIST 6 patients were from North Bengal Medical College and 9 patients were from IPGMER, Kolkata.
Results
Out of 15 registered patients diagnosed with GIST, 6 patients were male and 9 patients were female Figure 4. The median age was 45 years Figure 5. About 66.7% of patients were <50 years of age. Almost all had complaints of pain abdomen of chronic insidious onset which later aggravated. The mean duration of symptoms was 5.2 months Figure 6. Out of 15 cases, 3 cases had presented at the surgical emergency with the features of intestinal obstruction and perforation. Seven out of 15 (46.66%) of the cases were located in the stomach Figure 7 and rest in the intestines. Only one case had presented with omental metastasis probably of mesenteric origin and had liver metastasis.
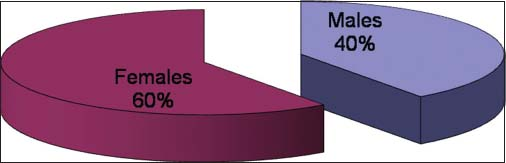
- Male:Female ratio
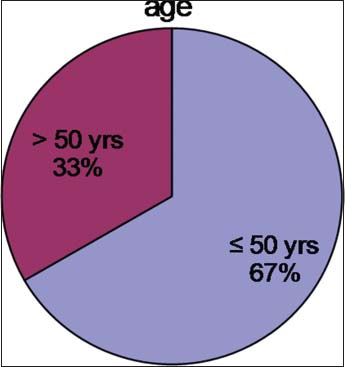
- Age distribution
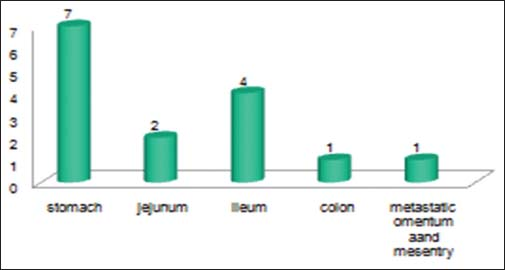
- Site wise location
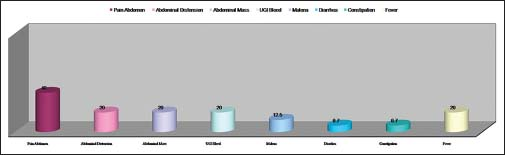
- Clinical presenting features
Primary surgery was possible for 14 cases and out of them, three cases were done as a surgical emergency (as mentioned earlier). A complete resection was possible in 9 cases and rest 5 cases had residual disease Figure 8. An open exploratory laparotomy biopsy was possible for the patient who had presented with metastatic disease. The postoperative histopathology and IHC markers were positive for c-kit in all cases. Only 33.335 cases were categorized as low-risk disease.
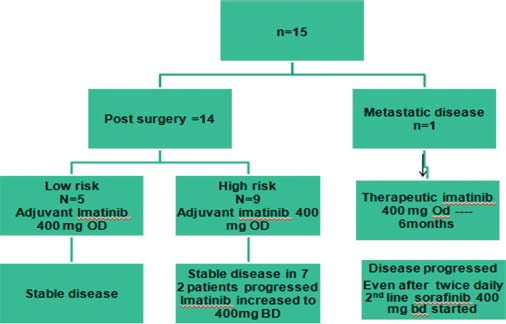
- Pattern of response to therapy
Imatinib was started for all patients at 400 mg once daily (OD) dosage. The evaluation was done at initial 1 month and then at 4 monthly and at 6 monthly periodic intervals. Imatinib was well tolerated, and there was no reported incidence of any rash, arthralgia, nausea, vomiting, or any psychiatric problem.
However, on evaluation at 6 months, three patients had progressive disease who were high risk stratified patients (1 metastatic at diagnosis and 2 other postoperatively who had residual disease). The dose of imatinib was increased from 400 mg OD to 400 mg BD for the all these 3 patients. After a median follow-up of 17.5 months, all low-risk patients have shown good response to imatinib with no evidence of relapse or recurrence. Out of all high-risk disease patients, 7 patients are still continuing on imatinib at 400 mg OD dose and 2 patients are on 400 mg BD dose. Unfortunately, the one patient who had metastasis and was taking imatinib 400 mg OD after disease progression at 6 months has progressed further, and now, a trial of sorafenib at 400 mg twice daily dose has been started 2 months ago. On evaluation, he has shown a partial response with respect to the liver lesions Figures 910. The Kaplan–Meier plot of 2-year actuarial overall survival (OS) and progression-free survival (PFS) are 79.12% and 86.67%, respectively Figures 1112.
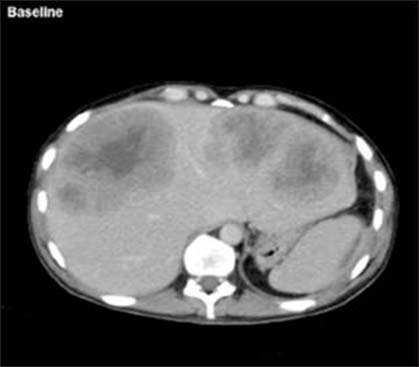
- Pretreatment liver metastasis
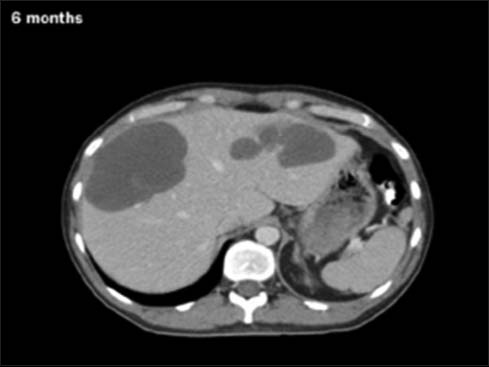
- Posttreatment partial regression
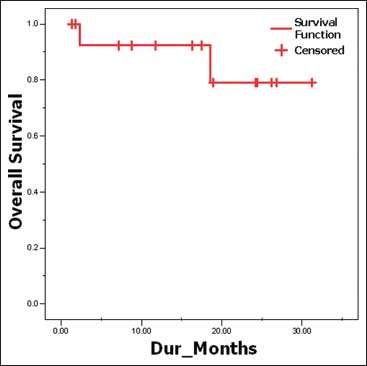
- Overall survival
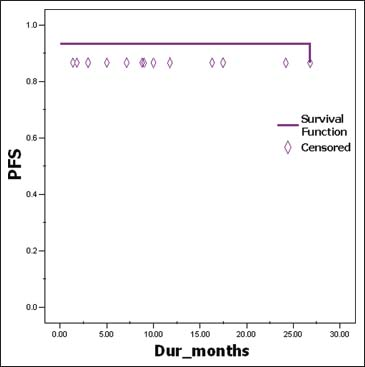
- Progression free survival
Discussion
Surgical resection has been the mainstay of treatment in localized disease. However, 40%–90% of patients have either postoperative recurrence or distant metastasis. Approximately 50% of patients have locally advanced or unresectable disease. The 5-year survival after complete resection is 50%–65% and in patients with advanced disease, it is 35%.7 Previously, there was no definite and effective nonsurgical therapy as GIST is insensitive to chemotherapy and radiation therapy.8
Imatinib is an effective systemic therapy for patients of GIST with good response rates and acceptable toxicity profile. The tumor size and mitotic index (no. of mitosis per high-power field [PHPF]) are two recommended prognostic factors which categorizes to high risk and low risk, respectively.9
Low risk tumor size <5 cm; and Mitotic rate – <5/PHPF.
High risk tumor size – >5 cm; >5 mitosis Per high power field.
-
>10 cm; any mitosis PHPF
-
Any size, >10 mitosis PHPF.
In this study, all patients had imatinib 400 mg OD as first line of therapy.
In the current study, imatinib has been found to be effective and well tolerated. Seven out of ten (70%) of the high-risk cases and 100% of low-risk cases have been observed to be effective on imatinib 400 mg OD dose. In an earlier study by Verweji et al. it was seen that there was no significant difference in response in dose 400 mg versus 800 mg with respect to complete response (5% in the 400 mg OD arm vs. 6% in the 800 mg OD arm), PFS and OS. Of late, the two large prospective randomized trials (EORTC-led intergroup 62,005 trial and the S0033 trial) had addressed this issue of 400 mg versus 800 mg. However, although the 400 mg dose still remains the gold standard both trials reported a superiority in terms of progression-free survival in the 800 mg arm, one reaching a significant statistical value (median progression-free survival 22 months vs. not reached; P = 0.02), the other being statistically nonsignificant (median progression-free survival 22 vs. 27 months; P = 0.13).10,11
Even though the first treatment with imatinib in a GIST patient was in 200012 neither its management at initial diagnosis nor the treatment of local and advanced disease has been standardized. The clinical practice has been generally based on the analysis of retrospective series of patients and prospective series with limited follow-up.
Hence, this current retrospective study is an endeavor to understand the patterns of presentation of GIST in two tertiary level hospitals and the management thereof. This study had its own limitations regarding short follow-up, few number of patients, lack of proper multidisciplinary approach, proper documentation, and protocol-based treatment.
A multidisciplinary consensus meeting has been held in 2004 in Lugano, Switzerland for the management of GIST and it involved 41 European, Asian, Australian, and American expert physicians, pathologists, molecular biologist, and surgeons under the auspices of ESMO. The goals were to identify a consensus as to the best practical approaches for the treatment of GIST, to summarize these conclusions in a written document, and to evaluate its impact on clinical practice.13
Conclusions
More and more studies and discussions will prove useful in the long run to address issues regarding quality assurance of c-kit IHC, targeting other markers, role PET scan as baseline, and assessing doubtful progression versus intratumoral bleed or nodule, timing of radiological interim analysis, duration of imatinib therapy in stable disease, and also understanding the types of resistance to therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The epidemiology of malignant gastrointestinal stromal tumors: An analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162-8.
- [Google Scholar]
- Prevalence and management of gastrointestinal stromal tumors. Am Surg. 2009;75:55-60.
- [Google Scholar]
- Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83.
- [Google Scholar]
- CD117: A sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728-34.
- [Google Scholar]
- Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-65.
- [Google Scholar]
- KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diagn Pathol. 2006;23:91-102.
- [Google Scholar]
- Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and stagingAnn Surg. 1992;215:68-77.
- [Google Scholar]
- Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: Differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211-20.
- [Google Scholar]
- Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608-15.
- [Google Scholar]
- Improved progression free survival in gastro-intestinal stromal tumors with high dose Imatinib. Results of a randomized phase III study of the EORTC, ISG and AGITGLancet. 2004;364:1127-34. et al.
- [Google Scholar]
- Continued prolongation of survival by imatinib in patients with metastatic GIST. Update of results from North American Intergroup phase III study S0033Proc Am Soc Clin Oncol. 2003;23:815. et al.
- [Google Scholar]
- Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-6.
- [Google Scholar]
- Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20-21 March 2004, under the auspices of ESMOAnn Oncol. 2005;16:566-78.
- [Google Scholar]







