Translate this page into:
Pancreatic Giant Cell Tumor: A Variant of Pancreatic Adenocarcinoma with Aggressive Course
Address for correspondence Venkata Pradeep Babu Koyyala, MD, Room: 2262, 2nd Floor, C Block, Rajiv Gandhi Cancer Institute and Research Centre, Sector 5, Rohini, New Delhi 110085, India. pradeepbabu.koyyala@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ductal adenocarcinoma of pancreas is the most common pancreatic neoplasm and is the third leading cause of death due to cancer, with a 5-year survival of 8% only. Although pancreatic neoplasms are broadly classified into endocrine and exocrine variety based on cell of origin, on imaging they are classified into solid and cystic types. Pancreatic giant cell tumor (PGCT) is a very uncommon and aggressive pancreatic neoplasm. We report a case of PGCT presenting as a pancreatic tail mass and liver lesions with a morphological overlap with other cystic benign and malignant lesions of pancreas on imaging.
Keywords
osteoclastic
pleomorphic
giant cell tumor
pancreas
Introduction
Ductal adenocarcinoma of pancreas is the most common pancreatic neoplasm and is the third leading cause of death due to cancer, with a 5-year survival of 8% only. Although pancreatic neoplasms are broadly classified into endocrine and exocrine variety based on cell of origin, on imaging they are classified into solid and cystic types. Pancreatic giant cell tumor (PGCT) is a very uncommon and aggressive pancreatic neoplasm. We report a case of PGCT presenting as a pancreatic tail mass and liver lesions with a morphological overlap with other cystic benign and malignant lesions of pancreas on imaging.
Case Report
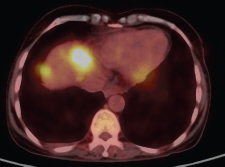
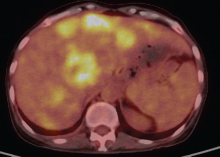
A 47-year-old woman who was housewife presented with epigastric pain, vomiting, and weight loss for past 4 months. There was no prior history of alcohol intake or smoking. Abdominal examination revealed an ill-defined mass in the epigastric region with mild tenderness. Laboratory results revealed normal amylase and lipase levels and elevated levels of carbohydrate antigen (CA) 19–9 serum marker and serum alkaline phosphatase. Diagnostic positron emission tomography–computed tomography (PET-CT) revealed an ill-defined metabolically active large lobulated solid/cystic soft tissue mass (11.1 × 9.8 × 16.0 cm, standardized uptake value [SUV] max 6.9) seen to arise from pancreatic tail region (Fig. 1). Metabolically active splenic venous thrombosis was seen (SUV max 9.3) reaching till confluence and extending into adjacent portal vein. Liver also revealed multiple metabolically active hypodense lesions in both lobes (largest in segment VIII 3.5 × 3.1 cm, SUV max 7.7) (Fig. 2). CT-guided biopsy yielded multiple fragmented soft tissue cores measuring 0.6 to 0.8 cm. Histopathologic examination (HPE) revealed infiltration by diffuse sheets of tumor cells having moderate eosinophilic to fine granular and vacuolated cytoplasm with indistinct borders, round vesicular nucleus, and prominent nucleoli. Fine fibrovascular septa were seen separating the tumor cells with occasional mitosis and areas of necrosis. Numerous multinucleated giant cells were seen. Overall the impression was that of a poorly differentiated neoplasm. Further immunohistochemistry characterization showed that tumor cells expressed CD68, CD163, and focal CK. These were negative for PAX-8, CEA, CK-7, CK-20, and synaptophysin, hence labeled as pleomorphic PGCT.
-
Fig. 1 Metabolically active splenic venous thrombosis was seen (SUV max 9.3) reaching till confluence and extending into adjacent portal vein.
-
Fig. 2 PET-CT showing multiple metabolically active hypodense lesions in both lobes of liver (largest in segment VIII 3.5 × 3.1 cm, SUV max 7.7). PET-CT, positron emission tomography–computed tomography.
In this patient, we were unable to start treatment due to very poor performance status of the patient. The patient succumbed within 15 days of presentation. This atypical case highlights the challenges in diagnosing pancreatic lesions and emphasizes the significance of considering less common forms of pancreatic tail masses and poor prognosis of variants of pancreatic carcinoma.
PGCTs are unusual nonendocrine tumors of the pancreas with prevalence less than 1% of all pancreatic tumors, first described by Sommers and Meissner in 1954.1 Three types of PGCT have been described based on histology: osteoclastic, pleomorphic, and mixed.2 Later in 2010, the World Health Organization (WHO) grouped them together as undifferentiated carcinoma with osteoclast-like giant cells.3 Clinical manifestations of pleomorphic giant cell carcinoma are analogous to those of pancreatic adenocarcinoma with abdominal pain and weight loss being the most prevalent.4 The survival range for pleomorphic giant cell pancreatic cancer ranges from several weeks in advanced unresectable disease to 25 months. Osteoclastic giant cell tumors may behave less aggressively than other PGCT.5 The lack of evidence upon which to base treatment decisions leads to difficulty in formative assessment of multidisciplinary approach for each patient.
Conflict of Interest
None declared.
References
- A case of osteoclast-like giant cell tumor of the pancreas associated with borderline mucinous cystic neoplasm. Pathol Oncol Res. 2009;15(01):129-131.
- [Google Scholar]
- Pleomorphic giant cell carcinoma of the pancreas with hepatic metastases—initially presenting as a benign serous cystadenoma: a case report and review of the literature. HPB Surg. 2010;2010:627360.
- [Google Scholar]
- Immunohistochemical and genetic analysis of osteoclastic giant cell tumor of the pancreas. Pancreas. 2006;32(03):325-329.
- [Google Scholar]
- Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer. 1977;39(05):2114-2126.
- [Google Scholar]
- Giant cell tumor of the pancreas of mixed osteoclastic and pleomorphic cell type: evidence for a histogenetic relationship and mesenchymal differentiation. Hum Pathol. 1990;21(11):1184-1187.
- [Google Scholar]







