Utilization and incorporation of tumor volume data in staging and prognostication of head and neck squamous cell carcinoma treated with definitive radiotherapy: A systematic review
Address for correspondence: Dr. Parveen Ahlawat, Department of Radiation Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector 5, Rohini - 110 085, New Delhi, India. drparveen7781@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Head and neck squamous cell cancers (HNSCC) are a group of heterogeneous tumors, evident by their diverse behavior and natural history. The largest diameter of tumor measured for T classification may not necessarily reflect the true tumor dimension. There is a need to take into account certain other feature(s) of these tumors other than the maximum single dimension which can reflect the true tumor burden more accurately. Tumor volume has been shown to be a useful and accurate tool burden because it is a measurement of tumor burden in all three dimensions. This review article has compiled and reviewed the literature published in past on impact of tumor volumes (TVs) on the prognosis of head and neck cancers. A comprehensive literature search was performed in PubMed for terms “clonogens,” “TV” or “primary TV (PTV)” or “nodal volume” or “total TV (TTV)” or “volumetric analysis of TV in head and neck” or “predicting response in head and neck cancer” “prognostic factors head and neck cancers” and “outcome in head and neck cancer.” We identified 33 studies which have commented on the impact of TV in HNSCC on treatment outcome, 9 of these had analyzed PTV, 11 studies had analyzed total nodal volume, and 14 studies have analyzed TTV. Besides these, we have dealt with laryngeal cancers separately with 9 studies. This review article is also aimed to enhance our knowledge further regarding how best a physician can incorporate TV data in staging and predicting response to radiotherapy.
Keywords
Head and neck cancer
prognosis
radiotherapy
tumor volume
Introduction
Head and neck cancers are the sixth most common cancer worldwide causing 300,000 head and neck cancer deaths annually globally.[1] Head and neck squamous cell cancers (HNSCC) are a group of heterogeneous tumors, evident by their diverse behavior and natural history.
American Joint Committee on Cancer uses tumor node metastasis (TNM) staging system and has been successful in bringing this diversity of HNSCC into clinical practice and use to predict prognosis. TNM staging system is indeed an expression of the anatomic extent of disease. T classification in TNM staging system typically measures the maximum single dimension of the primary tumor. This staging system is user-friendly and universally accepted for both, clinical use as well as for cancer research. However, it is felt that existing TNM staging system has certain limitations too. Head and neck cancers are three-dimensional lesions, they not only spread/infiltrate into different directions and planes within the head and neck region but also with nonuniform and unequal rate too, thus creating a complex shaped structure. Hence, the largest diameter of such a complex shaped structure measured for T classification may not necessarily reflect true tumor dimension. Figure 1 shows pretreatment scans of 5 patients from the author's institute illustrating variability in TV despite being same T-staged. Indeed, studies have shown that tumors belonging to same T classification may not necessarily have similar volume of disease, thereby reflecting the poor ability of T classification of TNM staging system in describing true dimensions of these heterogeneous class of tumors.[2,3] Another limitation of T classification of TNM staging system is that, it takes into account invasion or infiltration by primary tumor into some of those surrounding structures which are mainly important for the surgeon to decide on operability, but are of not much significance to nonsurgical treatment modality such as definitive radiotherapy (RT) or definitive concurrent chemoradiation (CCRT).
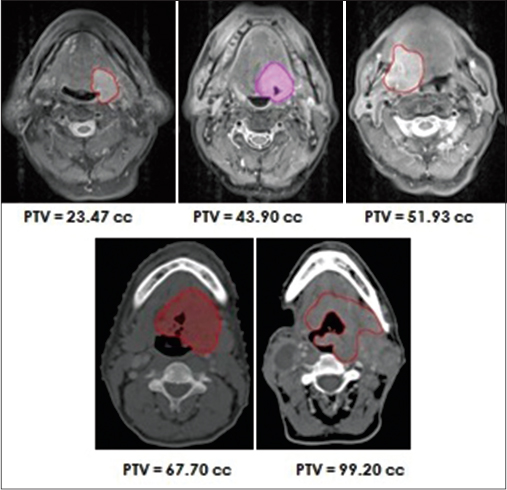
- Five patients with similar T-stage (T4a) but with different tumor volumes, showing variability in tumor volume within same T-stage
Having observed some of the limitations of TNM staging system there seem a need to find out certain other feature(s) of these tumors besides maximum single dimension which can more accurately reflect true tumor burden by taking into account all three dimensions of the tumor. While evaluating such feature(s) we must also ensure that we do not neglect the very vital information given by T classification of TNM staging system.
The aim of this review article is to compile and review the literature published in past on impact of tumor volumes (TVs) on the prognosis of head and neck cancers and also aimed to enhance our knowledge further regarding how best a physician can incorporate TV data in staging and predicting response to RT/CCRT.
Materials and Methods
A comprehensive literature search was performed in PubMed for terms “clonogens,” “TV” or “primary TV (PTV)” or “total nodal volume (TNV)” or “total TV (TTV)” or “volumetric analysis of TV in head and neck” or “predicting response in head and neck cancer” “prognostic factors head and neck cancers” and “outcome in head and neck cancer.” We identified 33 studies which have analyzed the impact of TV in HNSCC on treatment outcome, 9 of these had analyzed PTV, 11 studies TNV and 14 studies TTV. Besides these, we have dealt with laryngeal cancers separately with 9 studies.
Relationship between clonogens and tumor volume
There is considerable evidence that it is a single transformed cell which gives rise to cancer which in turn is evident by the experiments which have shown that same chromosomal abnormality is seen in every cell of cancer tissue. In order to completely sterilize a tumor by RT or, in other words, achieve cure requires that every single clonogenic cell capable of tumor growth is killed. Many authors have shown that the number of clonogenic cells increases almost linearly with an increase in tumor load and thus TV.[4,5] But it was Fletcher who first proved that there does exist a direct relationship between clonogen numbers and TV.[6] Hence, it seems that there exists a relationship between the probability of tumor response to RT and the TV. If the prognosis is to be estimated, an ideal but extremely cumbersome way is to count total initial clonogens. Another easier and practical way to do this is by measuring TV.
Johnson et al.[5] analyzed the data from a clinical trial of 51 patients with locally advanced head and neck malignancies treated by accelerated superfractionated RT. It was assumed that TV V, and clonogen number (m) are related by the equation:
m = α.Vb, where α is a proportionality constant.
Volume component b was estimated to be 0.85 (95% confidence interval [CI], 0.40–1.29), that is very close to unity. This finding by Johnson et al. proposed that TV and clonogen numbers have almost a linear relationship. This also makes an obvious and simple radiobiological inference that larger tumors have more clonogens and hence a larger tumor needs an increased dose of radiation to kill or sterilize all the clonogenic cells. Because there is a direct relationship between clonogen number, TV, and radiation dose, the TV can be utilized to predict the treatment outcome.
Although the discussion so far suggests that more the TV poorer the prognosis, very often in clinical practice it is not uncommon to find a cure in a large tumor and failure in a small tumor. This observation probably gives a clue that the relationship between tumor clonogen numbers, TV and prognosis may not hold good for a larger tumor. It may also be because there exist some other factors besides TV which contribute to the response to radiation therapy. Few such factors are cellular factors such as intrinsic radioresistance and oxygenation, etc.
Variability of tumor volume within same T-staged tumors
As discussed earlier, T-staging has the poor ability in describing heterogeneous tumors, tumors with similar T-staging but with different extent of invasion in different directions may have different TVs. Pameijer et al.[3] studied stage III larynx and hypopharynx primaries and observed the variability of TV (Vvol). Pretreatment computerized tomographic (CT) scans were taken of 42 patients with T3 head and neck carcinoma involving different subsites having distinct tumor boundaries were studied. Using these scans, TVs were measured using the summation of areas technique and Vvol was determined. Tumor volume measurements site wise were as follows: T3 larynx carcinoma Vvol, 1.7–17.0cc (median 3.7cc); T3 oropharynx carcinoma Vvol, 10.0–41.2cc (median 18.3cc); T3 hypopharynx carcinoma Vvol, 8.9–67.8cc (median 17.4cc); T3 nasopharynx carcinoma Vvol, 3.7–30.1cc; and T3 maxillary sinus carcinoma Vvol, 56.0–103.1cc. They found TVs of stage III larynx and hypopharynx carcinomas showing a highly significant variation (P = 0.0001). This could possibly be because the TNM system for most head and neck cancers are primarily based on the single dimensional extent of tumor or vocal cord fixation. In oropharyngeal carcinomas, such a strikingly difference was not observed within T3-stage. It was concluded that T3-staged tumors of the head and neck show considerable variability of TVs. This observation formed the very basic rationale of incorporation of TV data in further refining the TNM staging system for better prognostication of head and neck cancers.
Methods of measurement of tumor volume, imaging modalities, and related issues
Last decade witnessed a strongly emerging association of TV and disease outcome. In order to use TV as an independent factor, it is extremely important and vital that TV be measured accurately. Methods of measurements should be reliable and standardized. Obstacles in three-dimensional measurement of TVs and their routine use in clinical practice in past were two-dimensional conventional RT planning being the predominant method of treatment across the world, lesser availability of CT scan for RT purposes and variation in techniques of TV measurement.
Earlier, the process of TV measurement was tedious, involving outlining the tumor boundaries carefully and then by summation of area technique the volume was derived. Soon intra- and inter-observer variations in tumor outlining were realized doubting the reliability of these methods of TV measurement.[7,8,9] These days with the advancement and availability of modern imaging modalities, three-dimensional RT techniques and intensity modulated RT (IMRT) have become routine at most centers. Pretreatment contrast enhanced CT is the imaging of choice for treatment planning given its ability to provide intrinsic information on the electron density of the various tissues needed for dose calculation algorithms. The other advantage of CT is its growing availability. Motion artifacts caused by breathing, motion, coughing and swallowing, dental filling and other prosthesis, poor ability to differentiate tumor from edema are the main limitations with CT. Utilizing the opportunity that all patients undergo pretreatment contrast enhanced CT as a component of conformal RT, TV measurements are feasible and can be done easily. How significant is the inter-observer (variability in determining TV between observers) and intra-observer variability (variability in determining TV by the same observer on two different occasions), was evaluated by Hermans et al.[10] Five different observers determined laryngeal tumor for 13 tumors in four different sessions. There was significant variability found between observers (P < 0.0001) and between sessions (P < 0.01). It was inter-observer variability which accounted for 89% of the total variability. Thus, the variability can further be minimized if a single trained and experienced observer measures the TV. In order to overcome variability in TV measurement, several researchers have developed semi-automated or automated systems for tumor boundary outlining and TV measurement.[11,12,13] Even these techniques were criticized for their unproven validity. In recent years, computer-based automated or semi-automated tumor segmentation has been developed with an aim to minimize intra-observer and inter-observer variability.
Two more imaging modalities which seem to have been gaining importance in TV delineation and TV measurements are the magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT. MRI provide an excellent characterization of soft tissue compared with CT, but unlike CT, MRI does not provide electron density information. This limitation of MRI has been overcome to a great extent with the availability of registration and image fusion protocols in which MRI's diagnostic superiority can be combined with CT, thus enabling MRI for use in RT planning.[14] PET can also be used in radiation planning by importing it into the modern treatment planning software and co-registering it with planning CT scan. The volume of the tumor demonstrating fluorodeoxyglucose uptake is defined as metabolic tumor volume (MTV).[15] MTV represents a metabolic as well as a volumetric marker which estimates TV based on the distribution of metabolic activity. In other words, MTV quantifies the overall tumor burden.[16] PET-CT helps in improved target volume delineation by defining a metabolically active biological target volume.[17] Various studies have shown MTV to be a predictor outcome for certain cancers such as lung, esophageal cancer, and primary gastrointestinal B-cell lymphomas.[18,19,20] However, its role in predicting outcome in patients with head and neck carcinomas is not yet proved although various researches are underway.
Predicting early response evaluation using tumor volume data and its usefulness
The early disease response evaluation postchemoradiation in HNSCC has been realized as a difficult task owing to the posttreatment effects which hinder clinical and imaging (CT and MRI) findings such as delayed anatomical response in the tumor, distortions caused by early mucositis and late fibrosis. PET-CT also has been found to have poor positive predictive value to detect failure postchemoradiation owing to radiation-induced acute inflammation.[21,22] It should be the goal of any radiation oncologist to predict early treatment failure shortly after RT/CCRT in head and neck cancer patients and identify those early to whom the salvage surgery for cure could be offered at earliest for residual disease before it becomes surgically unresectable and radiation-induced fibrosis sets in making surgery difficult. How TV data can help in this decision was studied by Bhatia et al.[23] between 2001 and 2008 in 69 head and neck cancer patients performed MRI at diagnosis (pretreatment), 2 weeks during the CCRT and 6 weeks after CCRT and assessed early treatment outcome. Those who were found to have local failure (LF) had higher TV compared with those with local remission, at diagnosis (P = 0.01), 2 weeks during CCRT (P = 0.009) and 6 weeks after CCRT (P = 0.0001), thus concluding TV based on MRI 6 weeks post CCRT is most predictive of LF.
Impact of primary tumor volume on prognosis and prognostic “threshold cut-off” of primary tumor volume – all head and neck sites
Locally advanced head and neck cancers have a poor prognosis.[24,25,26] With the rapid advancement in the delivery of RT and the rapidly emerging era of organ preservation, CCRT is well-established treatment modality in locally advanced head and neck cancers. It is important to determine the predictors for outcome in these patients who are to be treated with CCRT. Until two decades, little was known about factors predicting the outcome for head and neck cancer treated with CCRT.
Last decade witnessed the plethora of evidence favoring impact of TV on the probability of local control (LC) after CCRT in locally advanced HNSCC. Many studies have shown that PTV adversely affects the treatment outcome [Table 1]. Pretreatment PTV has consistently been shown to serve as a better indicator of treatment response and outcome compared to classical TNM staging. Most studies we have gone through have outcomes such as 2, 3, and 5-year survival, 2-year distant metastases free survival (DMFS), disease-free survival (DFS) and local (regional) control. In general, both overall survival (OS) and local-regional tumor control deteriorates with an increase of the TV.
|
First author |
Primary |
Patients |
PTV median, range (cc) |
PTV cut-off value (cc) |
Endpoint |
P |
|---|---|---|---|---|---|---|
|
Pameijer[27] |
Early PFS |
23 |
NA |
6.5 |
LC |
0.021 |
|
Hermans[28] |
O |
112 |
NR (0.5-143.8) |
NR |
LC |
0.047 |
|
Doweck[29] |
OC, O, L, H |
64 |
NA |
19.6 |
LRR OS |
0.001 0.0007 |
|
van den Broek[30] |
OC, O, H, supraglottis |
92 |
NR (6.4-393.0) |
NR |
LC OS |
0.01 0.02 |
|
Plataniotis[31] |
OC, O, H, L |
94 |
14.7 (1.2-102.6) |
NR |
NR |
NR |
|
Tsou[32] |
H |
51 |
NA |
19.0 |
LC Survival |
0.001 0.036 |
|
Chen[33] |
H |
76 |
23.6 (3.8-152.4) |
30 |
3 years CSS 3 years RFS |
0.0001 0.0001 |
|
Strongin[34] |
O, H, L |
78 |
3.1-123.2 |
35 |
PFS OS |
0.004 0.001 |
|
Lok[35] |
O |
340 |
32.79 (4.1-306.63) |
32.79 (dichotomized at median) |
LC/LRC DMFS |
0.004 0.0008 |
PTV - Primary tumor volume; PFS - Progression-free survival; LRC - Loco-regional control; OS - Overall survival; LC - Local control; CSS - Cause-specific survival; RFS - Relapse-free survival; DMFS - Distant metastases free survival; NA - Not available; NR - Not reported; LRR - Loco-regional recurrence
Having established and concluded that TVs affect prognosis, the next more important question has been how best to utilize/incorporate this information objectively in predicting response in HNSCC after RT/CCRT? To do this most authors have determined an optimal prognostic “threshold cut-off” of TVs above and below which there is the greatest magnitude of difference in outcome, thus, categorizing patients into either favorable group (TV < cut-off) or unfavorable group (TV > cut-off). To determine this optimal cut-off value of TV receiver operating characteristics analysis method has been used by most. The area under the curve, the sensitivity, and the specificity have been calculated to analyze the diagnostic value of these cut-off values. Others have used a range of TV in cc (stratified in serially increasing order) to determine correlation with outcome. Few have dichotomized TV at the median and compared outcome with TV above and below the median. Following are the review of studies in order of their year of publication.
Pameijer et al.[27] identified a threshold cut-off of 6.5cc for PTV in 23 patients with T1 and T2 pyriform sinus primary treated with definitive RT. In patients with PTV <6.5cc LC rate was 89% and in those with PTV ≥6.5cc the LC rate was 25% (P = 0.021). This cut-off of 6.5cc predicted LC with a sensitivity of 94% and specificity of 60%.
Hermans et al.[28] in a retrospective study of 112 patients with oropharyngeal tonsillar cancer treated with curative intent by radiation therapy analyzed various CT based parameters such as PTV, TNV, nodal density and extracapsular extension on local and regional outcome and found a marginal significant correlation between PTV stratified according to volume quartiles and LC (P = 0.047). There was no significant correlation between PTV and LC within T2 (P = 0.57), T3 (P = 0.23), and T4 status (P = 0.14) when all patients were stratified according to median PTV. Being few number in patients in T1 category similar correlation between PTV and LC could not be seen.
Doweck et al.[29] in a retrospective analysis of 64 patients with locally advanced head and neck cancer treated with intra-arterial chemotherapy and RT showed PTV to be strongly correlated with local disease control and survival. PTV threshold of >19.6cc predicted greatest risk for LF (93.8% vs. 57%; P = 0.001). PTV was the only significant factor correlating with LF in nominal logistic regression analysis. Similarly, patients with PTV more than this cut-off demonstrated the survival of only 14.1% compared with 41.5% for those having PTV <19.6cc. Thus, PTV was found to be the most significant and independent factor correlating with survival in proportional hazard model (P = 0.0007).
van den Broek et al.[30] in a multivariate analysis of 92 patients with inoperable T3–T4 squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and supraglottic larynx treated with targeted chemoradiation (intra-arterial infusions of cisplatin) demonstrated PTV to be the only volume significantly associated with and predictive for LC (P = 0.01). It was observed that the probability of LC decreased by 2.6% with each 1cc rise in PTV. In multivariate analysis, PTV was found to be correlating with OS (P = 0.02).
Plataniotis et al.[31] in an attempt to explore the impact of tumor volumetry on outcome analyzed 94 patients with all sites of locally advanced HNSCC. Primary GTV was one of the significant factors found for the survival in univariate analysis. However, no prognostic threshold cut-off was identified.
Tsou et al.[32] in a retrospective study of 51 patients with stage III and IV hypopharyngeal primary tumors treated with definitive CCRT found PTV to be significantly correlating with local disease control and survival. Patients having PTV of >19.0cc had greatest risk for LF (P = 0.001). The survival rate significantly differed in patients having PTV >19.0cc and <19.0cc (39.3% and 78.3% respectively, P = 0.036). PTV was significantly associated with OS in proportional hazard model (P = 0.036). Univariate analysis showed TNV cut-off of 10.0cc as a poor prognostic factor with significant difference in LC above and below this cut-off (P = 0.029).
Chen et al.[33] studied 76 patients with stage III–IVA hypopharyngeal cancers treated with conventional RT (n = 30) and IMRT (n = 46). They found a PTV cut-off of 30cc. The 3-year cause-specific survival (CSS) was significantly higher in patients with PTV <30cc than with PTV >30cc (75% vs. 20%; P = 0.0001). Three years primary tumor relapse-free survival (PRFS) was significantly higher for those with a PTV <30cc than with PTV >30cc (72% vs. 23%; P = 0.0001). PTV <30cc versus >30cc was identified as a single prognostic factor for CSS in multivariate analysis (P = 0.0001, hazard ratio [HR] 2.84). Similarly, multivariate analyses of the PRFS showed similar finding, with a PTV >30cc (P = 0.0001, HR 2.55) being significant.
Strongin et al.[34] studied 78 patients with locally advanced (stage III and IV) hypopharyngeal, oropharyngeal and laryngeal carcinoma treated with definitive CCRT with an aim to evaluate the correlation between PTV and cancer control. They found interval to progression correlating well with PTV (P = 0.007). Patients with PTV <35cc had significantly better outcome than those with a PTV >35cc at 5 years (P = 0.010). Progression-free survival and OS were also better in patients with PTV <35cc (P = 0.004 and P ≤ 0.001 respectively). Furthermore, PTV was the best predictor of recurrence on multivariate analysis and survival (HR 4.7, 95% CI, 1.9–11.6; P = 0.001; HR 10.0, 95% CI, 2.9–35.1; P ≤ 0.001 respectively). Tumors having PTV larger than 21.6cc were associated with more locoregional failure (RF) (P = 0.028). Similarly, tumors having PTV larger than 27.1cc were associated with more distant metastasis (P = 0.020).
Lok et al.[35] in 340 patients with oropharyngeal cancers treated with definitive IMRT, analyzed the impact of PTV on OS, LF, RF and distant metastatic failure. The median PTV was 32.79cc (range, 4.10–306.63cc). Unlike other studies, the statistical approach used in this study was dichotomization of PTV at the median, that is, 32.79cc. The OS rate for PTV >32.79cc group was 82.7% (95% CI, 76.9–88.9%), whereas for PTV ≤32.79cc it was 94.3% (95% CI, 90.7–98.0%). On univariate analysis, this difference in OS rate was found to be significant, showing PTV to be a predictor of poor OS. On multivariate analysis PTV >32.79cc was shown to be associated with poor OS. The 2-year LF cumulative incidence rate for patients having PTV >32.79cc was 10.4% (95% CI, 5.7–15.1%) whereas for those having PTV ≤32.79cc it was 1.9% (95% CI, 0.0–4.1%). Similarly, the 2-year cumulative incidence rate in diabetes mellitus was 18.7% (95% CI, 12.6–24.8%) for PTV >32.79cc and 5.8% (95% CI, 2.1–9.6%) for PTV ≤32.79cc, which was further confirmed in multivariate analysis. This study demonstrated a significant relationship between PTV and local and distant control in the era of IMRT.
Impact of total nodal volume on prognosis and prognostic “threshold cut-off” of total nodal volume – all head and neck sites
As discussed earlier the use of the maximal diameter of regional lymph nodes and the number of involved nodes is not an accurate measure of the tumor load in the neck. The importance of the prognostic factor of TNV has been studied by many authors, but only few have described nodal volume as a separate entity in their studies. Table 2 summarizes studies which have evaluated nodal volumes and correlated with outcome. The reported threshold values of TNV lie in a relatively broad spectrum depending on the subsite of the disease. Most have found their cut-off values as significant in predicting the outcome. Three studies failed to show their “cut-off” for TNV being significant.[29,34,35]
|
First author |
Primary |
Patients |
TNV median and range (cc) |
TNV cut-off value |
Endpoint |
P |
|---|---|---|---|---|---|---|
|
Van den Bogaert[36] |
OC, O, H, L |
328 |
NR |
Volumetric stratification |
60 weeks survival |
0.016 |
|
Jakobsen[37] |
L, P |
280 |
NR (1-1413) |
100 |
Survival |
0.008 for larynx 0.002 for pharynx |
|
Hermans[28] |
O |
112 |
0-221.2 |
Volumetric stratification |
LC |
0.009 |
|
Doweck[29] |
OC, O, H, L |
64 |
7.8 (0.3-376) |
NR |
LC |
NS |
|
Plataniotis[31] |
OC, O, H, L |
94 |
3.7 (0-108.6) |
8.1 |
Survival |
0.044 |
|
Ljumanovic[38] |
OC, O, H, L |
311 |
10.5 (0.3-120) |
10.5 |
2 years DMFSR |
0.001 |
|
Vergeer[39] |
OC, O, H, L |
79 |
NR |
14.0 |
Regional control |
0.006 |
|
Tsou[32] |
H |
51 |
7.8 (0.5-80.9) |
10.0 |
LRC |
0.029 |
|
Chen[33] |
H |
44 |
16.2 (1.6-75.1) |
40 |
5 years LRFS |
0.001 |
|
Strongin[34] |
O, H, L |
78 |
0-437.66 |
NR |
PFS OS |
NS |
|
Lok[35] |
O |
128 |
19.04 (0-442.05) |
19.04 (dichotomized at median) |
RF |
NS |
TNV - Total nodal volume; NR - Not reported; LC - Local control; DMFSR - Distant metastasis-free survival rate; LRC - Loco-regional control; PFS - Progression-free survival; OS -Overall survival; RF - Regional failure; NS - Not significant; LRFS - Local relapse-free survival
Van den Bogaert et al.[36] in an earliest study done before the CT era, estimated nodal volume diameters determined by clinical examination for estimation of tumor and nodal volume in 328 patients and found a significant difference for median survival of 57 weeks (P = 0.016) in volumetric analysis for TNV.
Jakobsen et al.[37] studied 280 pharyngeal and laryngeal cancer with cervical lymphadenopathy treated with definitive RT at Centre for Head and Neck Cancer, Odense University Hospital. Patients were stratified into three volume categories (V1-V3): V < 10cc, V2 > 10cc - <100cc and V3 > 100cc resulting from univariate analysis of mean volume. Disease-specific survival was significantly related to V3 category for both laryngeal and pharyngeal cancer (P = 0.008 and P = 0.002, respectively).
Hermans et al.[28] in actuarial analysis of 112 patients with oropharyngeal tonsillar cancer treated with curative intent by radiation therapy and found a significant correlation between TNV stratified according to volume quartiles and LC (P = 0.009).
Doweck et al.[29] in a retrospective volumetric analysis of 64 patients with locally advanced head and neck cancer treated with intra-arterial chemotherapy and RT showed that TNV was not associated with loco RF.
Plataniotis et al.[31] studied 94 patients of head and neck cancer with nodal disease and identified a cut-off of 8.1cc for TNV. Median TNV for patients who were alive at the end of the study was 2.0 cc and for those who died was 8.1cc (P = 0.044), thus showing a significant difference in survival according to TNV.
Ljumanovic et al.[38] in a retrospective analysis done on pretreatment MRI of 311 patients with head and neck cancer, with 158 MRI positive cervical lymphadenopathy showed 2 years distant metastasis-free survival rate (DMFSR) of 94% for those without positive nodes and 75% for those with positive nodes. In univariate analysis, 2 years DMFSR was significantly higher in ipsilateral TNV <10.5cc than in >10.5cc (P = 0.001). Contralateral TNV <5cc was the only single factor found in the multivariate analysis to be predictive of 2 years DMFSR (HR: 13.9; 95% CI: 1.4–138.4; P = 0.02).
Vergeer et al.[39] demonstrated the negative prognostic effect of pathologic neck nodes. They evaluated a number of nodal features on pretreatment CT of 79 patients with head and neck cancer (all sites) treated with RT or CCRT. In univariate analysis, regional control was associated with TNV (P = 0.005). A TNV cut-off of 14cc was calculated, above and below which there was a significant difference in regional control at 2 years treated with CCRT (91% vs. 64%; P = 0.021). In multivariate analysis, TNV was one of the factors found significantly associated with the regional control.
Tsou et al.[32] in a retrospective study of 51 patients with stage III and IV hypopharyngeal primary tumors treated with definitive CCRT found TNV to be significantly correlating with local disease control. Univariate analysis showed TNV cut-off of 10.0cc as a poor prognostic factor with a significant difference in LC above and below this cut-off (P = 0.029).
Chen et al.[33] studied 44 patients with hypopharyngeal cancers. A TNV cut-off of 40cc was obtained. The 5 years local relapse-free survival was 75% for those with tumors <40cc and 26% when TNV was 40cc or greater (P = 0.001).
A study by Strongin et al.[34] found no significant correlation between the interval to progression and TNV. Also, TNV was not found to be a statistical significant prognostic factor. A possible reason for this unexpected result was very high nodal volume ranging from 0 to as high as >400cc and outliers having either absent nodal disease or having very large nodes which might have affected the statistical analysis.
Lok et al.[35] studied 340 patients with oropharyngeal cancers treated with IMRT and dichotomized TNV at a median of 19.04cc (0–442.05). Two years RF cumulative incidence of patients with TNV >19.04cc was 6.8% (95% CI, 2.9–10.7%) and of TNV ≤19.04cc was 3.7% (95% CI, 0.8–6.5%), however this difference was not found to be significant on univariate competing risks regression. Thus, this study could not prove a correlation between TNV and RF. The likely explanation for this nonsignificant relationship was the neck dissection which was assumed to be a confounding factor.
Impact of total tumor volume on prognosis and prognostic “threshold cut-off” of total tumor volume – all head and neck sites
TTV is sum total of PTV and TNV. Following is a review of studies which have demonstrated TTV to be a significant prognostic factor for disease outcome post RT/CCRT.
Johnson et al.[40] studied TTV in 76 patients (all sites) and found out a TTV cut-off of 35cc correlating well with DFS. Five years DFS was significantly better for volumes of <35cc (P = 0.0001).
Grabenbauer et al.[41] evaluated 87 patients with unresectable stage III and IV floor of the mouth, mobile tongue, the base of the tongue, tonsils, soft palate, and hypopharynx cancer. TTV of the primary and involved neck nodes was calculated. Analysis showed that TTV >110cc was predictive of 3 years survival rate in RT alone arm (5% vs. 53%) as well as in CCRT arm (22% vs. 69%; P = 0.0001). In multivariate Cox analysis TTV >110cc was associated with significantly better survival (P = 0.0008).
Rudat et al.[42] measured TTV in 56 inoperable advanced head and neck cancer patients treated with an accelerated simultaneous radiochemotherapy with carboplatin using a concomitant boost technique. A significant correlation between TTV and survival was demonstrated. DMFSR and OS differed significantly above and below TTV dichotomized at median that is, <112.3cc vs. >112.3cc (P = 0.05 and P = 0.003, respectively). A similar correlation was observed in the multivariate analysis also at same TTV (P = 0.0008, HR 3.0 (1.6–5.7).
Dietz et al.[43] in prospective trail including 25 patients with stage IV HNSCC of the oropharynx and hypopharynx. TTV ranged from 32.8 to 660.4cc (median 121.3cc). TTV did not correlate statistically significant with OS.
Hermans et al.[28] in the actuarial analysis of 112 patients with oropharyngeal tonsillar cancer treated with curative intent by radiation therapy and found no correlation found between TTV and locoregional outcome (P = 0.1).
Morris et al.[44] studied 133 oropharyngeal malignancy patients treated with accelerated hyperfractionated RT. LC rates were determined for <30cc versus >30cc divided by subsite. For oropharyngeal cancer, 2-year and 5-year locoregional control (LRC) rates correlated significantly with TTV (P = 0.003).
Kurek et al.[45] analyzed 107 patients retrospectively with head and neck cancer treated with CCRT and demonstrated TTV to be a prognostic factor. Results showed that with every increase in TTV by 10.0cc there was an increase in relative risk of death (RR = 1.006).
Dunst et al.[46] investigated 125 patients with head and neck cancer treated with RT and calculated TTV from pretreatment CT scan. TTV was strongly correlated with survival. TTV had a significant impact on 2-year OS (<32cc vs. >32cc; P = 0.024). Mean TTV was significantly higher in those died compared to those surviving (54cc vs. 34cc; P = 0.017). TTV was also found to be strongly correlating with prognosis in multivariate Cox regression model (P = 0.02). The impact of TTV on survival was demonstrated to be mainly resulting from the hypoxic volume. Nonhypoxic volume had no impact on survival.
Plataniotis et al.[31] studied 101 patients with head and neck cancer (all sites) and showed TTV to be an independent prognostic factor for survival in multivariate analysis. In patients who relapsed loco regionally within 3 years of follow-up the median TTV was 27cc (range, 1.3–153.3cc), and for those who did not relapse had median TTV 15.9cc (range, 1.3–72.6cc) (P = 0.017). Further, a prognostic threshold of 22.8cc was identified for TTV. Patients having TTV of <22.8cc had high likelihood of achieving complete response and median survival of 45.5 months, and patients having TTV of >22.8cc had a median survival of 12.3 months (P = 0.01), thus confirming TTV to be a significant prognostic factor.
Kuhnt et al.[47] investigated for the effect of prognostic effect of necrosis on the outcome. TTV was measured for 51 patients with locally advanced HNSCC treated with accelerated hyperfractionated RT with or without chemotherapy. Results showed that there was no significant impact of T TV on LC in multivariate analysis (P = 1.0).
Chufal et al.[48] calculated TTV (primary and involved neck nodes) on pretreatment high-resolution CT of 74 patients with head and neck cancer (all sites). TTV threshold cut-off of 25cc was identified to be predictive of OS. OS at 28 months for TTV <25cc and >25cc differed significantly (77.4% vs. 59.2%; P = 0.004).
La et al.[49] demonstrated in 85 patients with HNSCC the prognostic impact of MTV based on PET. There was an increase in hazard of first event with an increase in MTV of 17.4cc (difference between 75th and 25th percentiles) (recurrence or death, P ≤ 0.001).
Strongin et al.[40] studied 78 patients with locally advanced (stage III and IV) hypopharyngeal, oropharyngeal and laryngeal squamous cell carcinoma treated with definitive CCRT. Although PTV was found to be strongly correlating with the outcome, no such association was found between TTV. Possible reason for this unexpected result were very high nodal volume in a large range (0 to >400cc) contributing to TTV thus creating outliers having either absent nodal disease or having very large nodes which might have affected the statistical analysis.
Studer and Glanzmann[50] retrospectively studied 201 with T4-stage head and neck cancer patients and stratified them using volumetric staging system based on three cut-offs of TTV 15, 70, and 130cc, thus creating 4 subgroups for prognostic purpose. The subgroups were V1–V4: 1–15 ml (n = 15), 16–70 ml (n = 108), 71–130 ml (n = 62), >130 ml (n = 16). All underwent simultaneous integrated boost-IMRT with/without chemotherapy. Analysis showed that volumetric staging system (V1–V4) so formed was prognostic factor for OS: 90%/72%/58%/18%; DFS: 83%/50%/39%/10%; LRC: 81%/53%/47%/15%; DMFS: 93%/90%/70%/41%, all P < 0.0001. Table 3 summarizes the literature published in past correlating TTV and disease outcome.
|
First author |
Primary |
Patients |
TTV median, range |
TTV cut-off |
Endpoint |
P |
|---|---|---|---|---|---|---|
|
Johnson[40] |
P, L |
76 |
5-196 |
35 |
5 years DFS |
0.0001 |
|
Grabenbauer[41] |
OC, O, H |
87 |
NR |
110 |
LRC |
0.0008 |
|
Rudat[42] |
OC, O, P, L |
56 |
112.3 (16-660.4) |
112.3 (dichotomized at median TTV) |
OS |
0.0008 |
|
Dietz[43] |
O, H |
25 |
32.8-660.4 |
NR |
Survival |
NS |
|
Hermans[28] |
O |
112 |
NR |
NR |
LRC |
0.5 |
|
Morris[44] |
O |
133 |
1-240 |
30 |
2 years LRC |
0.003 |
|
Kurek[45] |
OC, O, P, L |
107 |
32.5 (2.1-220.1) |
NR |
Relative risk |
0.02 |
|
Dunst[46] |
OC, O, H, L |
125 |
NR (2-283) |
32 |
2 years OS |
0.024 |
|
Plataniotis[31] |
OC, O, H, L |
101 |
25.8 (1.3-153.3) |
22.8 |
OS |
0.01 |
|
Kuhnt[47] |
OC, O, H, L |
51 |
NR |
NR |
LC |
0.18 |
|
Chufal[48] |
OC, O, H, L |
74 |
29.6 (10.5-88.6) |
25 |
OS |
0.004 |
|
La[49] |
OC, O, H, L |
85 |
11.2 (0.8-88.9) |
NR |
DFS |
<0.001 |
|
Strongin[34] |
O, H, L |
78 |
NR |
NR |
PFS OS |
NS |
|
Studer[50] |
OC, O, H, L |
201 |
NR (7-216) |
Volumetric staging |
OS DFS LRC DMFS |
All<0.0001 |
TTV - Total tumor volume; NR - Not reported; DFS - Disease-free survival; LRC - Loco-regional control; OS - Overall survival; LC - Local control; PFS - Progression-free survival; DMFS - Distant metastasis-free survival; NS - Not significant
Most studies we have reviewed have found a correlation between TTV and outcome.
Impact of tumor volume on treatment outcome and prognostic “threshold cut-off” of tumor volume – a review specific on laryngeal cancers
Laryngeal tumors especially T1–T3 seem to have different natural history and differ from the other head and neck sites in various ways. They are generally low volume tumor by virtue of their small size in general and their lesser propensity for nodal involvement. Their T-staging (from T1 to T3) uses more of information on the invasion of structure within close vicinity of primary than the maximum single dimension of the tumor. For example, a 2 cm glottis tumor may be staged to T2 if vocal cord shows impaired mobility or growth involves and/or supraglottic subsite. Similar sized glottis and supraglottic tumor are upstage to T3 if fixed vocal cords are found. RT is generally preferred for T1 and T2 glottic tumors in view of better voice quality after voice sparing surgical techniques. T3 and T4 tumors confined to one side with CCRT or combined modality treatment have a better outcome than staged matched other sites of head and neck region. Considering these differences from other head and neck sites, it seems unfair to include small sized laryngeal tumors into head and neck region and study the impact of combined TV on prognosis. Hence, we have compiled and reviewed studies on impact of laryngeal TV and other head and neck sites (combined oral, oropharynx, hypopharynx and larynx) separately. This will give us an insight on their being low volume disease and lower “threshold cut-off” as compared to other sites of head and neck cancers. Table 4 summarizes the various publications in past favoring PTV and its correlation with disease outcome specially pertaining to laryngeal cancers.
|
First author |
Primary |
Patients |
PTV median, range (cc) |
PTV cut-off value (cc) |
Endpoint |
P |
|---|---|---|---|---|---|---|
|
Mukherji[51] |
Glottic |
28 |
NA |
NA |
LC |
NS |
|
Lo[52] |
Glottic, supraglottic |
55 |
NA |
4.0 |
LC |
<0.05 |
|
Hermans[53] |
Glottic |
61 |
NR (0.1-9.3) |
Volumetric classes |
LC |
0.0069 |
|
Hermans[54] |
Supraglottic |
103 |
NR (0.1-139.6) |
NR |
LC |
<0.001 |
|
Hamilton[55] |
Glottic, supraglottic |
47 |
NR (0.2-16.64) |
3.0 for both sites |
LC |
0.003 |
|
1.0 for glottic |
LC |
0.001 |
||||
|
Pameijer[56] |
Glottic |
42 |
NR |
3.5 |
LC |
0.0002 |
|
Mancuso[57] |
Supraglottic |
63 |
NA |
6 |
LC |
0.001 |
|
Kraas[58] |
Supraglottic |
28 |
3.1 (0-68.6) |
6.0 |
LC |
0.07 |
|
8.0 |
LC |
0.007 |
||||
|
Mendenhall[59] |
Glottic, supraglottic |
404 |
NA |
NA |
LC |
0.0042 |
PTV - Primary tumor volume; NR - Not reported; NA - Not available; NS - Not significant; LC - Local control
Mukherji et al.[51] in an attempt to determine whether pretreatment CT predict LC in early glottis cancer (T2), retrospectively evaluated 28 patients treated with RT alone. This study found no association between TV and disease outcome. The probable reason for this result could be the role of certain other tumor-host biological factor(s) in such low volume diseases which were not detectable on CT, but influenced the outcome. This study is probably the only negative study where the relationship between TV and outcome was not seen in laryngeal cancers.
Lo et al.[52] in a retrospective study analyzed 55 patients with T2 and T3 glottic and supraglottic carcinoma treated with RT or surgery. Results showed that PTV cut-off of 4.0cc was a predictor of LF in T2 laryngeal cancer only treated with RT (P < 0.05). PTV did not correlate with LC in neither T3 cancers nor in combined all T-stage cancers.
Hermans et al.[53] studied many CT-derived parameters in 119 glottis cancers, one amongst them was PTV. PTV was stratified into classes in following the order: <1cc, 1–2cc, 2–4cc, 4–8cc and 8–10cc. A significant correlation was found between PTV classes and LC within the glottic T1 category (61 patients) (P = 0.0069).
Hermans et al.[54] evaluated influence of various CT-determined parameters such as PTV, TNV, TTV and pattern of local extension on local and locoregional outcome of 103 patients with supraglottic cancer treated with definitive RT. PTV stratified in serially increasing volume classes were found to have a significant correlation with LC (P = 0.0002). The actuarial analysis revealed PTV to be significantly correlated with LRC (P < 0.001). Univariate analysis of TTV was statistically significant with P = 0.0016 (divided by <2cc, 2–4cc, 4–8cc, 8–16cc, 16–32cc, 32–64cc). In multivariate analysis TTV was the strongest independent indicator of LRC in supraglottic carcinoma (P = 0.0032).
Hamilton et al.[55] in a retrospective analysis of 47 patients with T2 and T3 glottic (n = 30) and supraglottic (n = 17) cancer treated with definitive RT, showed LC to be significantly correlating with TV threshold of 3cc for both sites combined (>3cc vs. <3cc; P = 0.003) and TV threshold of 1cc for glottis cancers (>1cc vs. <1cc; P = 0.001) in multivariate analysis. It was also demonstrated that T-stage of the primary did not correlated with the local recurrence rate.
Pameijer et al.[56] evaluated 42 patients of T3 glottis carcinoma to determine if pretreatment CT scan can predict LC treated with definitive RT. PTV was found to be a significant predictor for the outcome. A threshold cut-off of 3.5cc was determined above and below which there was a significant difference in LC (25% vs. 85%; P = 0.0002).
Mancuso et al.[57] Studied 63 patients with T2–T4 supraglottic cancer treated with definitive RT alone and found LC rates to be inversely proportional to TV. A threshold TV of 6cc was identified, above and below which there was a significant difference in LC (52% vs. 89%; P = 0.0012). The result was also confirmed in multivariate analysis.
Kraas et al.[58] studied 28 patients with supraglottic carcinoma treated with definitive RT. Median PTV was 3.1cc (range, 0–68.6cc). A PTV cut-off of 8cc was identified above and below which there was a significant difference in the outcome. In follow-up period ranging from 20 to 58 months LC rate was 20% for patients with PTV >8cc and 70% for those with PTV <8cc (P = 0.0077). When stratified PTV at 6cc no significant association was found between TV and LC rate at 2 years (67% and 43%: P = 0.07).
Mendenhall et al.[59] in a multivariate analysis showed that LC was significantly influenced by PTV in glottis and supraglottic primaries (P = 0.0042 and P = 0.0220 respectively). PTV was found to have more significant influence on LC than T-stage (P = 0.0220 vs. 0.2791) for glottis and supraglottic.
Conclusions
Head and neck tumors are three-dimensional lesions with the unequal rate of tumor spread in different directions and different planes. Hence, the largest diameter of tumor as suggested by T classification in TNM staging system need not reflect the total tumor burden of this disease. TV measurement is a better and more accurate reflection of the true total tumor burden or extent of the disease. With the advances in radiologic imaging and three-dimensional treatment planning, TV data and measurements are easy to obtain. Since most patients undergo pretreatment CT and/or MRI, no extra cost is needed to measure TVs. There is variability in the TVs within same T classification in locally advanced HNSCC. If the prognosis is to be estimated it is simply not enough to measure a largest single dimension of the tumor. It is concluded and strongly claimed that TV, namely PTV, TNV, and TTV are powerful predictors of treatment outcome in HNSCC treated with definitive RT/CCRT and should be taken into account in prognostication. There is a need for newer methods of staging by which overall prognosis of a disease can be determined more accurately but without losing the very vital anatomical extent of disease information given by TNM system. One such method is the incorporation of TV data into already existing TNM staging. TV staging system or derived prognostic cut-off values of TVs can be brought into routine clinical practice for staging and prognostication purposes. For example, a patient with base of tongue carcinoma, clinically staged as cT3N1M0 with PTV 25cc, can be even more accurately staged as cT3N1M0 (PTV 25cc) or cT3N1M0 (PTV below/above cut-off). If TVs are to be used clinically for prognostication purposes, it is vital that that the methods of volume measurement be more standardized and reliable across the world, so as to have true and meaningful exchange and comparison of results. Reviewing this plethora of evidence favoring the role of TV in prognostication of HNSCC we suggest that it is the time that specialized large burden cancer centers start incorporating TV data into routine use for staging and prognostic purpose. One area which warrants further investigation and research is to bring up consensus for one such particular “prognostic threshold cut-off” value for TV (PTV, TNV and TTV) which can be utilized by all physicians so as to bring uniformity and better exchange of information.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Correlation between MR imaging-derived nasopharyngeal carcinoma tumor volume and TNM system. Int J Radiat Oncol Biol Phys. 2006;64:72-6.
- [Google Scholar]
- Variability of tumor volumes in T3-staged head and neck tumors. Head Neck. 1997;19:6-13.
- [Google Scholar]
- Dose, volume, and tumor-control predictions in radiotherapy. Int J Radiat Oncol Biol Phys. 1993;26:171-9.
- [Google Scholar]
- The tumor volume and clonogen number relationship: Tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys. 1995;33:281-7.
- [Google Scholar]
- Basic clinical parameters In: Fletcher G H, ed. Textbook of Radiotherapy. Philadelphia, PA: Lea and Febiger; 1980. p. :180-219.
- [Google Scholar]
- Radiotherapy response of cerebral metastases quantified by serial MR imaging. J Neurooncol. 1994;21:171-6.
- [Google Scholar]
- A quantitative assessment of the addition of MRI to CT-based, 3-D treatment planning of brain tumors. Radiother Oncol. 1992;25:121-33.
- [Google Scholar]
- Laryngeal tumor volume measurements determined with CT: A study on intra- and interobserver variability. Int J Radiat Oncol Biol Phys. 1998;40:553-7.
- [Google Scholar]
- MR tissue characterization of intracranial tumors by means of texture analysis. Magn Reson Imaging. 1993;11:889-96.
- [Google Scholar]
- Unsupervised measurement of brain tumor volume on MR images. J Magn Reson Imaging. 1995;5:594-605.
- [Google Scholar]
- Application of fuzzy c-means segmentation technique for tissue differentiation in MR images of a hemorrhagic glioblastoma multiforme. Magn Reson Imaging. 1995;13:277-90.
- [Google Scholar]
- New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79:S2-15.
- [Google Scholar]
- Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861-8. et al.
- [Google Scholar]
- Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527-34.
- [Google Scholar]
- Challenges in integrating 18FDG PET-CT into radiotherapy planning of head and neck cancer. Indian J Cancer. 2010;47:260-6.
- [Google Scholar]
- Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer. 2012;13:52-8.
- [Google Scholar]
- Postchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:471-7.
- [Google Scholar]
- Prognostic value of metabolic tumor volume on PET/CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477-82.
- [Google Scholar]
- Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo) radiotherapy in head and neck cancer. Head Neck. 2005;27:175-81.
- [Google Scholar]
- The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001-10.
- [Google Scholar]
- Does primary tumour volumetry performed early in the course of definitive concomitant chemoradiotherapy for head and neck squamous cell carcinoma improve prediction of primary site outcome? Br J Radiol. 2010;83:964-70.
- [Google Scholar]
- Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: A phase II trial of the radiation therapy oncology group (RTOG 99-14) Int J Radiat Oncol Biol Phys. 2008;71:1351-5.
- [Google Scholar]
- Competing causes of death in patients with locoregionally advanced head and neck cancer treated with concomitant boost radiation plus concurrent weekly cisplatin. Clin Transl Oncol. 2013;15:321-6.
- [Google Scholar]
- Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol. 2011;6:70.
- [Google Scholar]
- Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20:159-68.
- [Google Scholar]
- The relation of CT-determined tumor parameters and local and regional outcome of tonsillar cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2001;50:37-45.
- [Google Scholar]
- Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope. 2002;112:1742-9.
- [Google Scholar]
- Pretreatment probability model for predicting outcome after intraarterial chemoradiation for advanced head and neck carcinoma. Cancer. 2004;101:1809-17.
- [Google Scholar]
- Prognostic impact of tumor volumetry in patients with locally advanced head-and-neck carcinoma (non-nasopharyngeal) treated by radiotherapy alone or combined radiochemotherapy in a randomized trial. Int J Radiat Oncol Biol Phys. 2004;59:1018-26.
- [Google Scholar]
- Analysis of prognostic factors of chemoradiation therapy for advanced hypopharyngeal cancer – Does tumor volume correlate with central necrosis and tumor pathology? ORL J Otorhinolaryngol Relat Spec. 2006;68:206-12.
- [Google Scholar]
- Prognostic impact of tumor volume in patients with stage III-IVA hypopharyngeal cancer without bulky lymph nodes treated with definitive concurrent chemoradiotherapy. Head Neck. 2009;31:709-16.
- [Google Scholar]
- Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1823-30.
- [Google Scholar]
- Intensity-modulated radiation therapy in oropharyngeal carcinoma: Effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851-7.
- [Google Scholar]
- The EORTC randomized trial on three fractions per day and misonidazole in advanced head and neck cancer: Prognostic factors. Radiother Oncol. 1995;35:100-6.
- [Google Scholar]
- Lymph node metastases from laryngeal and pharyngeal carcinomas – Calculation of burden of metastasis and its impact on prognosis. Acta Oncol. 1998;37:489-93.
- [Google Scholar]
- Distant metastases in head and neck carcinoma: Identification of prognostic groups with MR imaging. Eur J Radiol. 2006;60:58-66.
- [Google Scholar]
- Control of nodal metastases in squamous cell head and neck cancer treated by radiation therapy or chemoradiation. Radiother Oncol. 2006;79:39-44.
- [Google Scholar]
- The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995;32:635-41.
- [Google Scholar]
- Nodal CT density and total tumor volume as prognostic factors after radiation therapy of stage III/IV head and neck cancer. Radiother Oncol. 1998;47:175-83.
- [Google Scholar]
- Prognostic impact of total tumor volume and hemoglobin concentration on the outcome of patients with advanced head and neck cancer after concomitant boost radiochemotherapy. Radiother Oncol. 1999;53:119-25.
- [Google Scholar]
- Prognostic assessment of sonography and tumor volumetry in advanced cancer of the head and neck by use of doppler ultrasonography. Otolaryngol Head Neck Surg. 2000;122:596-601.
- [Google Scholar]
- Accelerated superfractionated radiotherapy with concomitant boost for locally advanced head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2002;52:918-28.
- [Google Scholar]
- Usefulness of tumor volumetry as a prognostic factor of survival in head and neck cancer. Strahlenther Onkol. 2003;179:292-7.
- [Google Scholar]
- Tumor volume and tumor hypoxia in head and neck cancers. The amount of the hypoxic volume is importantStrahlenther Onkol. 2003;179:521-6.
- [Google Scholar]
- Impact of tumor control and presence of visible necrosis in head and neck cancer patients treated with radiotherapy or radiochemotherapy. J Cancer Res Clin Oncol. 2005;131:758-64.
- [Google Scholar]
- Analysis of prognostic variables among patients with locally advanced head and neck cancer treated with late chemo-intensification protocol: Impact of nodal density and total tumor volume. Jpn J Clin Oncol. 2006;36:537-46.
- [Google Scholar]
- Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335-41.
- [Google Scholar]
- Volumetric stratification of cT4 stage head and neck cancer. Strahlenther Onkol. 2013;189:867-73.
- [Google Scholar]
- Can pretreatment CT predict local control of T2 glottic carcinomas treated with radiation therapy alone? AJNR Am J Neuroradiol. 1995;16:655-62.
- [Google Scholar]
- Tumour volume: Implications in T2/T3 glottic/supraglottic squamous cell carcinoma. J Otolaryngol. 1998;27:247-51.
- [Google Scholar]
- Predicting the local outcome of glottic squamous cell carcinoma after definitive radiation therapy: Value of computed tomography-determined tumour parameters. Radiother Oncol. 1999;50:39-46.
- [Google Scholar]
- Value of computed tomography as outcome predictor of supraglottic squamous cell carcinoma treated by definitive radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:755-65.
- [Google Scholar]
- Computed tomographic volumetric analysis as a predictor of local control in laryngeal cancers treated with conventional radiotherapy. J Otolaryngol. 2004;33:289-94.
- [Google Scholar]
- Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy? Int J Radiat Oncol Biol Phys. 1997;37:1011-21.
- [Google Scholar]
- Preradiotherapy computed tomography as a predictor of local control in supraglottic carcinoma. J Clin Oncol. 1999;17:631-7.
- [Google Scholar]
- Quantitative analysis from CT is prognostic for local control of supraglottic carcinoma. Head Neck. 2001;23:1031-6.
- [Google Scholar]
- Parameters that predict local control after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2003;25:535-42.
- [Google Scholar]







