Translate this page into:
Tailored approach to management of bilateral breast cancer in Indian women
Address for correspondence: Dr. Veda Padma Priya Selvakumar, Department of Surgical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi - 110 085, India. privedsri@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction: Bilateral breast cancer BCC is relatively uncommon with an overall incidence of 5–20% in women with early breast cancer. They are divided into synchronous if cancers are detected simultaneously or within 6 months of each other and metachronous if they are detected more than 6 months apart from each other. Family history and hereditary cancers multicentricity and lobular histology are some of the factors associated with BCC. In this background, we sought to evaluate the incidence, clinicopathological profile, and management of women with bilateral primary breast cancer at our institute.
Materials and Methods: We retrospectively reviewed the medical records of women who underwent surgery for BCC at the breast services unit at our institute from October 2010 to April 2015. The clinicopathological profile and outcomes were analyzed using SPSS 22 software and appropriate statistical tests.
Results: Out of 1330 women who underwent surgery for early breast cancer between October 2010 and April 2015, 44 were bilateral. Twenty-eight were synchronous and 16 were metachronous. Mean age of the presentation of patients was 53 years (range 30–79 years). The histological type were same in 82.14% of synchronous tumors and 87.5% of metachronous tumors (P = 0.496). The grades were similar in 42.85% of synchronous tumors and 56.25% of metachronous lesions (P = 0.294). The stage concordance among synchronous tumors was 39.28%, whereas it was 60% among metachronous lesions (P = 0.164).
Conclusions: The management of BCC is complex and has to be tailored to the individual based on characteristics of index and second tumor, prior therapy, adjuvant treatment, and risk stratification. Moreover, the concordance of receptor expression is higher in synchronous cancers than metachronous cancers.
Keywords
Bilateral breast cancer
metachronous breast cancer
synchronous breast cancer
Introduction
Bilateral breast cancer (BCC) is relatively uncommon with an overall incidence of 5–20% in women with early breast cancer. They are divided into synchronous if cancers are detected simultaneously or within 6 months of each other and metachronous if they are detected more than 6 months apart from each other. Family history and hereditary cancers multicentricity and lobular histology are some of the factors associated with BCC. In this background, we sought to evaluate the incidence, clinicopathological profile, and management of women with bilateral primary breast cancer at our institute.
Materials and Methods
We retrospectively reviewed the medical records of women who underwent surgery for BCC at the breast services unit at our institute from October 2010 to April 2015. The clinicopathological profile and outcomes were analyzed using SPSS statistics Version 22 (IBM) software and appropriate statistical tests.
Results
Forty-four confirmed cases of second primary breast cancer in the contralateral breast were encountered over 4 years period in 1330 women with invasive breast cancer treated in our department of whom 16 patients had metachronous and 28 patients had synchronous BBC. Mean age of the presentation of patients was 53 years (30–79 years).
Mean age of the presentation of synchronous BBC patients was 55 years (35–79 years). Most patients had early-stage disease on the contralateral side compared with the index side. Six patients received neoadjuvant chemotherapy for locally advanced breast cancer. Seventeen patients had bilateral mastectomy, 2 patients had bilateral breast conserving therapy, and 9 patients had combination of breast conservation and mastectomy.
Twenty-two women had invasive ductal carcinoma (IDC) on both sides; one had lobular carcinoma on both sides. Five women had IDC on one side and ductal carcinoma in situ (DCIS) on other side. On histopathological evaluation, the grade was similar on both sides in 12 patients (Grade I - 2, Grade II - 7, Grade III - 3). Bilateral Nodal positivity was seen in 4 patients. 14 patients had similar stage on both sides Table1. Twenty-five patients received adjuvant chemotherapy, and 3 patients underwent neoadjuvant chemotherapy. Fourteen patients received unilateral radiation and 6 received bilateral adjuvant radiation and 23 patients received adjuvant hormonal therapy. Four patients were triple negative on both sides, 1 patient was triple positive on both sides, and 12 patients were estrogen receptor/progesterone receptor (ER/PR) positive and human epidermal growth factor receptor 2 (HER2)/neu negative on both sides. ER/PR and HER2/neu were discordant in 11 patients. At a mean follow-up of 21 months, all patients were disease free and mean overall survival of patients with small bowel cancer is 100% Table 2.
|
Variable |
Synchronous (n=28) |
Metachronous (n=16) |
||
|---|---|---|---|---|
|
First tumor |
Second tumor |
First tumor |
Second tumor |
|
|
Age at diagnosis (years) (median (range)) |
55 (35-79) |
41.9 (27-58) |
50.1 (30-73) |
|
|
Stage |
||||
|
0 |
1 |
4 |
2 |
0 |
|
I |
2 |
3 |
0 |
1 |
|
II |
16 |
18 |
12 |
13 |
|
III |
9 |
3 |
2 |
2 |
|
Histological type |
||||
|
IDC |
26 |
23 |
14 |
16 |
|
DCIS |
1 |
4 |
2 |
0 |
|
ILC |
1 |
1 |
0 |
0 |
|
Histological grade* |
||||
|
1 |
3 |
5 |
0 |
0 |
|
2 |
10 |
14 |
5 |
10 |
|
3 |
14 |
5 |
9 |
6 |
IDC - Invasive ductal carcinoma; DCIS - Ductal carcinoma in situ; ILC - Invasive lobular carcinoma
|
Bilateral breast |
Total n |
Number of events |
n |
Percentage |
|---|---|---|---|---|
|
Metachronous |
16 |
0 |
16 |
100.0 |
|
Synchronous |
28 |
0 |
28 |
100.0 |
|
Overall |
44 |
0 |
44 |
100.0 |
Mean age of the presentation of metachronous breast cancer was 50 years (30–75 years). Most patients had early-stage disease on the contralateral side compared with the index side. The duration of presentation of the second primary breast cancer ranged from 14 months to 20 years. Five patients received neoadjuvant chemotherapy. Eight patients underwent bilateral mastectomy, 1 patient underwent bilateral breast conserving therapy Figure 1, and 6 patients underwent breast conservation on one side and mastectomy on the other side.

- Magnetic resonance mammogram of synchronous bilateral breast cancer. Who underwent bilateral breast conservation
On HPE, 14 out of 16 patients were found to have IDC on both sides, 2 of them had DCIS on 1 side, and IDC on other. The grade was similar on both sides in 11 patients (Grade II - 6, Grade III - 5) Table 1. Eleven patients received adjuvant chemotherapy, and 1 patient did not receive chemotherapy. Four patients had unilateral and 6 had bilateral adjuvant radiotherapy and 14 patients received hormonal therapy. One patient was triple negative on both sides, and 2 patients were ER/PR positive and HER2/neu negative on both sides. At a mean follow-up of 64 months, all patients were disease-free and overall survival of patients with MBC is 100% Table 2.
The histological type were same in 82.14% of synchronous tumors and 87.5% of metachronous tumors (P = 0.496). The grades were similar in 42.85% of synchronous tumors and 56.25% of metachronous lesions (P = 0.294). The stage concordance among synchronous tumors was 39.28%, whereas it was 60% among metachronous lesions (P = 0.164) Table 3 and Figure 2.
|
Synchronous (%) |
Metachronous (%) |
P |
|
|---|---|---|---|
|
Histological type |
82.14 |
87.5 |
0.496 |
|
Grade |
42.85 |
56.25 |
0.294 |
|
Stage |
39.28 |
60 |
0.164 |
|
Receptor |
67.85 |
50 |
0.198 |
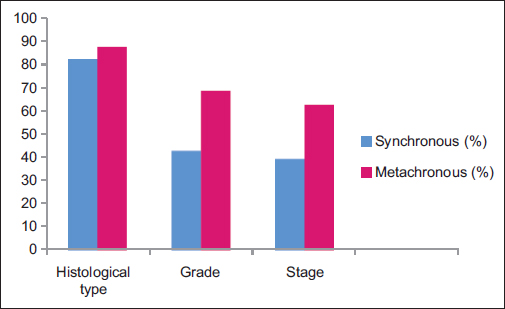
- Pathological concordance of bilateral breast cancer
Discussion
BCC is encountered either in a synchronous fashion or in a metachronous fashion. In both settings, the second lesion is either a metastatic lesion or a second primary. Chaudary et al. proposed the following criteria for the diagnosis of the second primary breast cancer in 1984:8
-
Presence of in situ change in the contralateral breast
-
The tumor in the second breast is histologically different from the primary tumor
-
The degree of histological differentiation in the second breast is distinctly greater than the lesion in the first breast
-
There is no evidence of local, regional, or distant metastases from cancer of the ipsilateral breast.
The introduction of cDNA microarray-based comparative genomic hybridization in the 90s utilized DNA copy number changes to differentiate between synchronous and metachronous lesions. In metachronous lesions, the numbers of DNA copy number changes are higher than synchronous cancers.9 However, because it is complicated and expensive, Chaudary's criteria still hold clinical relevance. The only caveat of Chaudary's criteria is that synchronous cancers are considered metastatic than multifocal or clonal.
The incidence of BCC in our institute during the study period was 3.3% (44/1330). In accordance with the literature, metachronous cancers affected younger women at the time of the first diagnosis,1 whereas synchronous lesions affected older women (mean age at presentation was 55 years).2
A plausible explanation for the difference in age may be attributed to the longer life expectancy of younger women with favorable tumors who are therefore at a higher risk of developing a second breast cancer.3 The receptor concordance in the synchronous group is 67.85% and 50% in the metachronous group (P = 0.198). The ER concordance in the synchronous group was 64.28% and 50% in the metachronous group. On the other hand, PR concordance in the synchronous group was 64.28% and 43.75% in the metachronous group. It is notable that the ER and PR concordance is similar in the synchronous group, while it is different in the metachronous group. The HER2/neu concordance is 57.14% in the synchronous group and 18.75% in the metachronous group Figure 3.
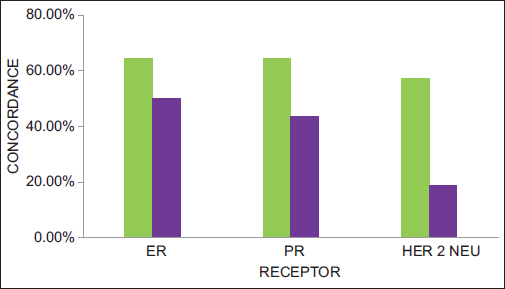
- Receptor concordance of bilateral breast cancer
Kheirelseid et al. reported a histological concordance of 79.2% and ER concordance of 49.5% in bilateral cancers.4,12 Renz et al. demonstrated a histological concordance of 54.8% and ER and PR status concordance of 86.2% and 79.3%.5
Huo et al. evaluated 30,617 women with BCC between 1990 and 2006 and found out a strong association in ER status between bilateral tumors with OR of 7.64 and concluded that concordance in hormone receptor status between BCCs in the same patient suggest that both the tumors arise in a common milieu and that their subtypes are predetermined early in the process of carcinogenesis.6,7
Sighoko et al. reported the observed discordance in ER status between two BCCs was the highest in metachronous contralateral pairs (28.0%) and the lowest in synchronous ipsilateral pairs (7.0%).10
Gong et al. found out that the concordance rates of histopathological type, T stage, TNM stage, and PR expression were higher in synchronous cancers than metachronous cancers (P = 0.032).11
Conclusions
The management of BCC is complex and has to be tailored to the individual based on characteristics of index and second tumor, prior therapy, adjuvant treatment, and risk stratification. Moreover, the concordance of receptor expression is higher in synchronous cancers than metachronous cancers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Evolving perspectives in contralateral breast cancer. Eur J Cancer. 1998;34:2000-9.
- [Google Scholar]
- Prognostic significance of synchronous and metachronous bilateral breast cancer. World J Surg. 2001;25:1117-24.
- [Google Scholar]
- Bilateral breast carcinoma: Clinical characteristics and its impact on survival. Breast J. 2010;16:625-32.
- [Google Scholar]
- Synchronous and metachronous bilateral breast cancer: A long-term single-institution experience. Med Oncol. 2012;29:16-24.
- [Google Scholar]
- The contralateral synchronous breast carcinoma: A comparison of histology, localization, and magnetic resonance imaging characteristics with the primary index cancer. Breast Cancer Res Treat. 2010;120:449-59.
- [Google Scholar]
- Concordance in histological and biological parameters between first and second primary breast cancers. Cancer. 2011;117:907-15.
- [Google Scholar]
- Receptor characteristics of the second tumor in synchronous versus metachronous breast cancer. Am Surg. 2008;74:702-5.
- [Google Scholar]
- Bilateral primary breast cancer: A prospective study of disease incidence. Br J Surg. 1984;71:711-4.
- [Google Scholar]
- The pattern of gene copy number changes in bilateral breast cancer surveyed by cDNA microarray-based comparative genomic hybridization. Int J Mol Med. 2004;13:17-24.
- [Google Scholar]
- Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: Biological difference or misclassification? Oncologist. 2014;19:592-601.
- [Google Scholar]
- Bilateral breast cancer: Differential diagnosis using histological and biological parameters. Jpn J Clin Oncol. 2007;37:487-92.
- [Google Scholar]
- Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat. 2011;126:131-40.
- [Google Scholar]







