Translate this page into:
Simultaneous Modulated Accelerated Radiotherapy (SMART) with Dysphagia Aspiration-Related Structures (DARS) Sparing: Do We Have a Role for Dose Condensation in Locally Advanced Head and Neck Cancer
Address for correspondence Bindhu Joseph, MD, Department of Radiation Oncology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka 560029, India. bindhu271@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction Concurrent chemotherapy integrated with intensity-modulated radiotherapy (IMRT) is the standard of care for locally advanced head and neck cancer. Simultaneous integrated boost technique has allowed differential doses to primary and normal structures permitting significant toxicity reduction. The current study explores the feasibility of the simultaneous modulated accelerated radiotherapy (SMART) technique to enhance cytoreduction and explore the possibility of dose intensification with radiobiologically targeted treatment condensation.
Methods Thirty patients were randomized in an open-labeled study to receive concurrent chemoradiation of 60 Gy in 25 fractions with “SMART” technique or 70 Gy in 35 fractions using conventional intensity-modulated radiotherapy simultaneous integrated boost “IMRT SIB.” The primary endpoints included comparative volumetric cytoreduction between the study and control arm assessed during the course of treatment and final response evaluation. Secondary endpoints involved the assessment of acute toxicity parameters for xerostomia, mucositis, dysphagia, and fatigue.
Results The “SMART” study arm showed comparable volumetric cytoreduction to the conventional “IMRT SIB” arm at midtreatment (p-value = 0.225) as well as toward completion (p-value = 0.476). The study arm did observe 94.4% cytoreduction of tumor volume compared with 88.05% in the conventional arm at the time of response evaluation. In spite of treatment condensation, there was no significant increase in toxicity with “SMART.” There was no difference in the frequency or duration of grade 3 mucositis in the “SMART” arm in spite of intensification (p-value = 0.728). In the “SMART” arm, there was a favorable reduction in the duration of grade ⅔ dysphagia; 2.8 weeks versus 4.6 weeks (p-value = 0.002). Even though the xerostomia was comparable in frequency and intensity, the total duration of xerostomia was 50% less (p-value = 0.001).
Conclusions The “SMART” technique provides a radiobiologically sound, effective, and safe protocol that has the potential to improve the treatment of locally advanced head and neck cancer. The good tolerability and toxicity profile in the study arm is encouraging and facilitates further research.
Keywords
simultaneous modulated accelerated radiotherapy
intensity-modulated radiotherapy
simultaneous integrated boost
chemoradiation
locally advanced head and neck cancer
Introduction
Head and neck cancers form the sixth most common cancer worldwide.1 The Global Cancer Observatory (GLOBOCAN) 2018 worldwide cancer statistics report indicates 834,860 head and neck cancers annually, while mortality is around 431,131.2 The majority of patients with head and neck cancer present with locally advanced disease.2 Concurrent chemotherapy integrated with conformal radiotherapy has become the standard of care for the management of patients ineligible for surgical treatment or planned organ preservation.1 This was established by a meta-analysis of over 17,000 patients from 93 trials.3,4 However, survival statistics for locally advanced head and neck cancer (LAHNC) remain dismal at 40 to 50% at 5 years with conventional doses of 70Gy.5,6 With modern advances in radiation technology, most centers have the provision of delivering conformal treatment to the tumor with favorable sparing of normal tissues both in terms of xerostomia and dysphagia.7,8 Now that there is scope for normal tissue sparing, the current focus of research is directed at tilting the benefits of the therapeutic ratio by treatment condensation. In our study, we hope to achieve this by combining the benefits of selective dose escalation to the target with “SIB” along with treatment condensation to 5 weeks. In this study, patients will be treated to a dose of 60Gy in 25 fractions (Eq. D2–62Gy) with the simultaneous integrated boost simultaneous modulated accelerated radiotherapy (SIB SMART) technique, thus providing an advantage of reducing overall treatment time by 16 to 18 days. Accelerated repopulation of tumor clonogens has been postulated as a possible cause of treatment failure.9,10 This anticipated event usually occurs around the third to fifth week and addressing this period with treatment intensification may provide us with a window of opportunity to improve local control.11,12 This could either be done with timed dose escalation or a reduction in the overall treatment time. The study arm with “SMART” hopes to achieve this through the latter, while still providing a radiobiologically equivalent dose to the high-risk tumor volumes.13,14 Using the linear quadratic (LQ) model, there is a considerable discrepancy between the two arms 84Gy10 versus 74.4Gy. However, when using the Fowler's equation, the reduced tumor proliferation and overall treatment time of the study arm were incorporated, after which the difference in biologically effective dose (BED) was dramatically reduced.11 After the inclusion of anticipated repopulation in the LQ model, the BED of both the study and control arm was comparable (65.9–76.2Gy) versus (66.1–73 Gy).
BED for 70Gy/35 Fractions
70(1 + 2/10) = 84Gy
The following equation takes into account the repopulation and the loss of dose as calculated by Fowler's formula.
BED = nd(1 + d/[α/β]) - loge2(T-Tk)/αTp
-
T—overall treatment time
-
Tk—kick off time
-
Tp—potential doubling time
70(1 + 2/10) – 0.693/0.3*(46–21)/5= 84–11.6Gy = 72.4Gy
BED for 60Gy/25 Fractions
60(1 + 2/10) – 0.693/0.3*(33–21)/5 = 74.4–5.082Gy = 69.3Gy
After accounting for the repopulation, the BED calculated (Tk—21days, Tp—5days [has a range of 2–25 days] α = 0.3/Gy) is comparable. In an earlier similar study done by Tandon et al (where they had compared similar dose per fractionations), a range of BEDs was calculated for each arm, according to the time of initiation of accelerated repopulation.15 The range of BEDs was 65.9 to 76.2Gy10 and 66.1 to 73Gy10 for the control and study arms, respectively.15 We, therefore, concluded that the two fractionation schedules used could be considered as having a similar BED for the tumor tissue.
The primary objective of this study would be to evaluate comparative volumetric cytoreduction between the two arms at 40 Gy (midtreatment), at the end of treatment, and at the time of response evaluation (6 weeks post-treatment). We would also be assessing acute toxicity in terms of intensity and duration of the most common dose-limiting anticipated side effects namely mucositis, dysphagia, xerostomia, and fatigue. The secondary objectives would be response evaluation between both arms at 6 weeks post-treatment.
Materials and Methods
This was a randomized open-labeled clinical study approved by the Clinical Ethics Board. The random allocation was made using computer-designed random numbers. Thirty patients were randomized to receive 60 Gy in 25 fractions with “SMART” (15 patients) or “IMRT” of 70Gy in 35 fractions (15 patients). Both study and treatment arm would be receiving concurrent weekly cisplatin at a dose of 40 mg/m2. The inclusion criteria for the current study were patients with squamous cell carcinoma of the oropharynx, hypopharynx, or larynx who were eligible for concurrent chemoradiation. This included patients aged between 18 and 70 years with a good performance status (Eastern Cooperative Oncology Group ≤ 2 and Karnofsky performance status >60). Patients with primary tumor volume that was less than 3 cm in greatest dimension or those having a second primary or a previous history of irradiation were excluded from the study. All patients fulfilling these inclusion criteria, registered in the Department of Radiation Oncology, Kidwai Memorial Institute of Oncology from January 1 2019 to June 30, 2020, were enrolled into the study. Patient parameters including age, sex, tumor site, histology, comorbidities, and history of substance abuse were recorded. Written informed consent was taken from all the patients.
Treatment Planning
Patients were simulated with contrast using Philips large bore CT (Philips Medical Systems, Cleveland, Ohio, United States) simulator scan.
Contouring and Target Volumes
All the patients were contoured so as to have the clinical target volume (CTV1) to include the gross tumor volume (GTV) and 5 mm margin for primary disease cropped to anatomical borders. The CTV2 included high-risk areas and was separately created with a 1 cm margin for primary disease. The CTV2 and CTV3 for nodal regions represented areas of high-risk and low-risk microscopic disease, respectively. The standard treatment arm (control) would receive a dose prescription of 70, 63, and 56 Gy to the high risk (CTV1), intermediate risk (CTV2), and low risk (CTV3).16,17 For the study arm, the corresponding values for CTV1, CTV2, and CTV3 were 60, 55, and 50Gy, respectively. Dysphagia aspiration-related structures was contoured as per the guidelines defined by Christianen et al.18 The superior pharyngeal constrictor was contoured from the caudal tip of the pterygoid plate till the lower edge of the C2 vertebra, middle pharyngeal constrictor from the upper edge of C3 vertebra till the lower edge of the hyoid bone, and inferior constrictor from the lower edge of hyoid till the lower edge of the cricoid cartilage. The esophageal inlet muscles and cervical esophagus were contoured in a single volume from the caudal edge of cricoid cartilage up to the sternal notch and the base of the tongue from the lower edge of the anterior tubercle of the atlas to the upper edge of the hyoid bone. The larynx, the supraglottic larynx and glottis were contoured separately. The supraglottic larynx was defined from the tip of the epiglottis till the upper edge of the arytenoid cartilage and the glottic larynx was contoured from the upper edge of arytenoid cartilage till the lower edge of the cricoid cartilage. The organs at risk (OARs), namely bilateral parotid glands, mandible, and spinal cord, were contoured according to the guidelines.19 All the patients were planned with Monaco treatment planning systems version: 5.11.02 and treated on an Elekta Infinity/Versa HD machine. In the study group, all the patients were planned with dynamic arc IMRT and received 60 Gy in 25 fractions to the primary planning target volume (PTV) and nodes greater than 3 cm in size. They were planned to receive 55 Gy in 25 fractions to the microscopic disease and 50 Gy in 25 fractions to the low-risk PTV.
The dosimetric constraints applied included a mean dose of 63Gy to the superior and middle pharyngeal constrictor muscle when treating oropharyngeal primaries and this was not considered when hypopharynx was involved. The mean dose to the inferior pharyngeal constrictor muscle was restricted to 56 Gy.20 The constraint for the oral cavity was a mean dose of 30Gy. Each individual parotid was planned to receive a mean dose of less than 26 Gy. The point dose to the spinal cord was not to exceed 45Gy. These constraints were uniform as the intermediate-dose and low-dose volumes received conventional dose per fraction in the “SMART” arm.
Assessment of Primary and Secondary Objectives
Patients in both arms underwent a volumetric radiological assessment for tumor cytoreduction at preplanned intervals using a simulation computed tomographic scan. The planned intervals for the same were at 40Gy, end of treatment, and at 6 weeks post-treatment. The patients were evaluated for acute toxicity related to mucositis, dysphagia, fatigue, and xerostomia according to NCI CTCAE v4.21 Descriptive and inferential statistical analysis has been performed in the present study. The descriptive and inferential statistics were performed using the Student's t-test. Descriptive statistics of categorical variables were reported in total numbers and percentages. Continuous variables were reported as mean, median, and standard deviation. p-Value <0.05 was considered statistically significant. The Statistical software SPSS 22.0 was used for the analysis of data.
Results
Both arms of the current study were comparable in terms of clinical characteristics as shown in Table 1
|
Gender |
Study |
Control |
|---|---|---|
|
Male |
13 |
14 |
|
Female |
2 |
1 |
|
Stage |
||
|
II |
0 |
0 |
|
III |
9 |
4 |
|
IV |
6 |
10 |
|
T Status |
||
|
T1,T2 |
1 |
2 |
|
T3,T4 |
14 |
13 |
|
N Status |
||
|
≤ N2a |
13 |
0 |
|
≤ N2b |
7 |
3 |
|
Nodal involvement |
||
|
Node absent |
3 |
1 |
|
Node present |
12 |
14 |
|
Site |
||
|
Oropharynx |
12 |
13 |
|
Hypopharynx |
2 |
0 |
|
Larynx |
1 |
2 |
|
Comorbidities |
||
|
Nil |
13 |
12 |
|
Present |
2 |
3 |
|
Pathology |
||
|
Grade 1 |
2 |
2 |
|
Grade 2 |
7 |
4 |
|
Grade 3 |
3 |
3 |
|
Grade not specified |
3 |
6 |
|
Substance abuse |
||
|
Chewed tobacco |
||
|
Yes |
13 |
12 |
|
No |
2 |
3 |
|
Smoking |
||
|
Yes |
13 |
12 |
|
No |
2 |
3 |
|
Alcohol |
||
|
Yes |
10 |
12 |
|
No |
5 |
3 |
Thirty patients were enrolled in this study. The median age of patients was 55 years (35–69). The majority of the patients (95%) had T3/T4 staged primaries with nodal involvement. Patients were staged according to the eighth edition of tumor nodes metastases. All the patients enrolled had excellent compliance and were able to complete treatment without undue treatment breaks defined as 5 days or greater continuous break. Only one patient in the study arm died with aspiration during treatment. However, he did not have significant mucositis so the cause of aspiration could not be attributed to toxicity. Seventy-five percent of patients were able to complete at least five cycles of concurrent cisplatin-based chemotherapy at 40 mg/m2. Patients were evaluated for the primary endpoint that is relative cytoreduction of GTV at three endpoints, midtreatment, at the end of treatment, and at 6 weeks subsequently. The pattern and extent of cytoreduction are depicted in Fig. 1A.
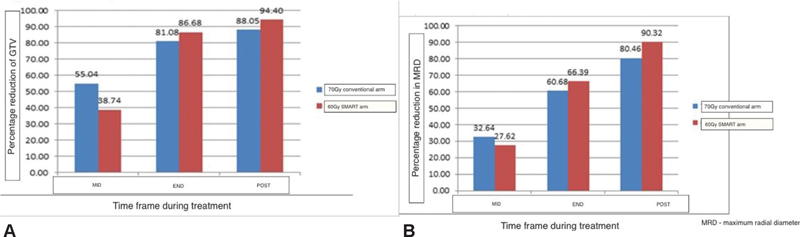
-
Fig. 1 (A) Mean percentage reduction in gross tumor volume (GTV). (B) Mean percentage reduction in tumor diameter. SMART, simultaneous modulated accelerated radiotherapy.
In both arms, there was a nearly 50% reduction of primary tumor bulk (GTV). The control arm showed a marginally better cytoreduction at midweek 38.74 versus 55.04%; however, toward the end of treatment, this was comparable. At 6 weeks post-treatment, the “SMART” technique showed a better response with a mean percentage cytoreduction of 94.1% versus 88.055 in the conventional arm (p-value = 0.86).
A radiological complete response was seen in four patients of the study arm and six patients in the control arm. To substantiate volumetric data, we also evaluated two-dimensional radial parameters in terms of cytoreduction. The values observed were comparable to the volumetric data and suggested the consistency of the same.
The maximum reduction in the diameter was observed toward the end of treatment with the “SMART” arm showing more reduction of 66.39 versus 60.08%. The greater reduction in tumor dimensions persisted at 6 weeks postradiotherapy and reached 90.37% in the study arm compared with only 80.46% in the conventional control.
Assessment of Toxicity Parameters
Xerostomia
In the current study, patients were evaluated for parotid-related toxicity in terms of xerostomia that was also correlated to the objective parameter of parotid shrinkage (volumetric cytoreduction). No patient in either arm developed grade 3 xerostomia. Grade 2 xerostomia was observed in all patients in both arms. Earlier studies by Sanguineti et al had documented the correlation of parotid shrinkage with the rate of xerostomia. In his study, greater midtreatment parotid shrinkage was directly associated with a higher occurrence of xerostomia.22 In the current study, patients in the protocol arm had a midtreatment shrinkage of 15% that was 10% less than the control arm. However, shrinkage at the end of treatment and at 6 weeks post-treatment was comparable. Although the objective criteria of parotid shrinkage did not reflect in increased xerostomia, it may have a correlation with the duration of grade 2 xerostomia compared with the conventional treatment group. Patients receiving “SMART” had a significantly shorter mean duration of 2 weeks xerostomia with a faster recovery compared with 5 weeks in the control arm (p-value = 0.001). The pattern of parotid shrinkage is shown in Fig. 2A and B.
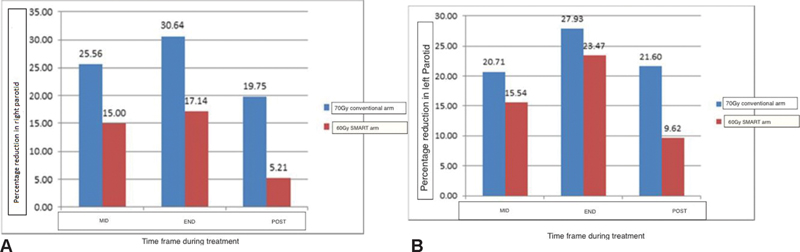
-
Fig. 2 (A) Mean percentage shrinkage in volume of the right parotid. (B) Mean percentage shrinkage in volume of the left parotid. MRD, maximum radial diameter; SMART, simultaneous modulated accelerated radiotherapy.
The pattern of occurrence, duration, and recovery of xerostomia is diagrammatically represented in Fig. 3.
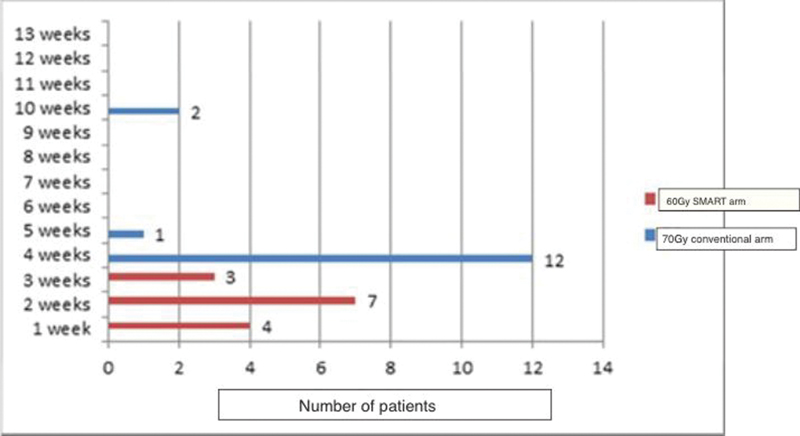
-
Fig. 3 Xerostomia duration in weeks. SMART, simultaneous modulated accelerated radiotherapy.
Mucositis and Dysphagia
Mucositis has been identified universally as the most important dose-limiting toxicity that can compromise treatment completion. In the current study, treatment was well tolerated in both arms. Although nearly all the patients had grade 3 mucositis (92% in the study arm and 93% in the control arm), only one patient in the study arm required persistent assisted feeding with Ryle's tube for one additional week post-treatment. The pattern of grade 3 mucositis in both arms is depicted in Fig. 4.
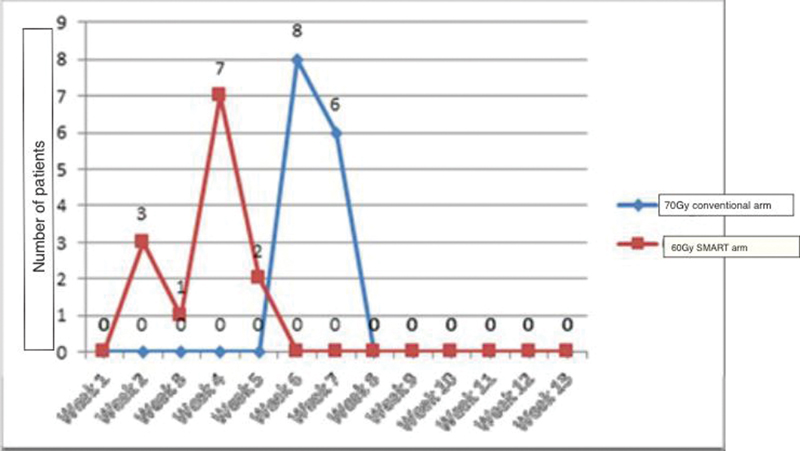
-
Fig. 4 Onset of mucositis (grade 3) in study arm versus the control arm. SMART, simultaneous modulated accelerated radiotherapy.
As observed in Fig. 4, the onset of grade 3 mucositis was significantly earlier in the protocol arm. Patients receiving “SMART” developed mucositis significantly earlier than those in the control arm receiving conventional treatment. The mean duration of onset of grade 3 mucositis in the study arm was 3 versus 6 weeks in the control arm (p-value = 0.001). The relative duration of grade3 mucositis in both arms is depicted in Fig. 3. The majority of patients in both arms had a duration of grade 3 mucositis limited to 2 weeks.
Dysphagia
Thirty-six percent of patients in both arms achieved pharyngeal constraints, while 52% superior and middle constrictor constraints. The mean deviation from the desired constraints was only 3.9% for superior and middle constrictors and 4.3% for the inferior constrictor. The majority of patients in both arms tolerated treatment well. Although all patients had grade 2 dysphagia starting from the 2nd week, only one patient who was in the conventional 70Gy arm progressed to grade 3 dysphagia and required Ryle's tube feeding. The pattern of dysphagia in terms of onset closely correlates with the development of mucositis. Most of the patients experienced difficulty in swallowing 1 week prior to the observation of grade 3 dysphagia. The earlier onset of dysphagia in the study arm also mimics the pattern of mucositis observed. The mean time period of observation of dysphagia in the study arm was 2.5 versus 4 weeks in the control (p-value = 0.001). Interestingly even though mucositis appeared earlier in the study arm, it also resolved faster. The mean duration of grade2 dysphagia in the study arm was 2.8 weeks, nearly 40% shorter than the control arm that was 4.6 weeks (p-value = 0.002).
Fatigue
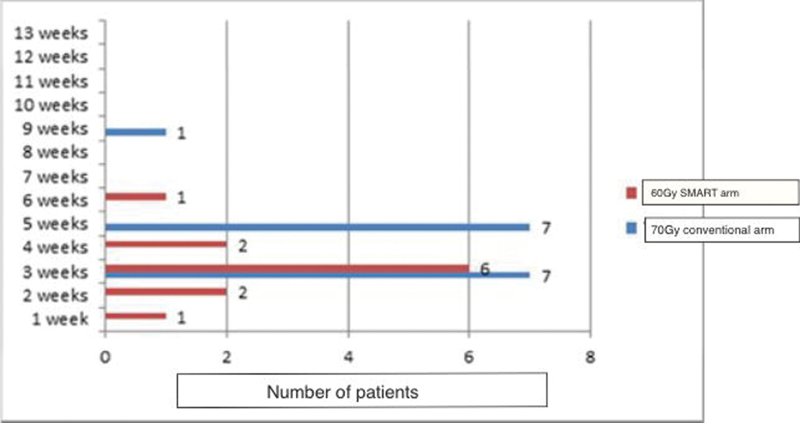
In the study arm, the incidence of fatigue and its duration was significantly lesser than what was observed in the protocol arm. Grade 2 fatigue was observed in 11 patients (78.5%) of the study arm versus 15 patients (100%) in the control arm (p-value = 0.005). The mean duration of fatigue was also significantly less in the study arm 2 versus 4 weeks in the control arm (p-value = 0.005). The duration and pattern of grade 2 fatigue observed for both arms in this study are depicted in Fig. 5.
-
Fig. 5 Duration of fatigue. SMART, simultaneous modulated accelerated radiotherapy.
Discussion
The concept of “SMART-SIB” in LAHNC was proposed in 1996 by Butler et al.14 He established the safety of this regimen and supported the potential benefit of selective dose escalation and treatment time condensation in providing a radiobiological advantage. To bring this concept into the current standard of care viz concurrent chemoradiation, Tandon et al conducted a prospective randomized trial to evaluate the tolerance, feasibility, and efficacy of this regimen when compared with the standard protocol with conventional doses.15 The use of weekly cisplatin has a comparative control rate and better toxicity profile than the 3 weekly regimens and that is why it has been incorporated in the current trial.23,24 One of the problems often faced in using weekly cisplatin is the inability to attain a cumulative dose of 200 mg/m2. However, in our study both the arms tolerated this component of treatment with 65% of the study arm and 80% of the control arm is able to receive at least five cycles of concurrent chemotherapy.
The main intention of this study was to try to quantify the radiobiological benefit of selective dose escalation through serial radiological assessment and documentation of tumor cytoreduction. It was observed that the protocol arm did have a trend toward enhanced tumor cytoreduction 86.6 versus 81.08% in the control arm. Although this did not achieve statistical significance (p-value = 0.86), it is promising and worth evaluating with a larger cohort of patients. The benefit of reduction of overall treatment time by 2 weeks was most evident in the pattern of normal tissue response. Although we had anticipated a marginal increase in acute toxicities in the study arm, it was not evident in the current protocol. The complete treatment was well tolerated in the study arm and comparable in terms of compliance and toxicity with the control arm receiving conventional treatment. There were no significant treatment breaks on account of toxicities and all patients were able to complete the protocol.
Xerostomia, both acute and late, is one of the most distressing side effects of head and neck radiotherapy in terms of quality of life.25 The dose escalation in the study arm did not increase the rate of acute xerostomia in comparison to the control. Patients in both arms had the same chances of grade 2 xerostomia with none progressing to grade 3. Sanguineti et al in an earlier study had postulated that maximum midtreatment parotid shrinkage could predict the chances of late xerostomia.22 The study arm had an advantage over the control arm with a 10% lesser rate of shrinkage. We will have to wait for a longer follow-up to see if this extrapolates to clinically less xerostomia. More importantly, the protocol arm showed a shorter duration of observed xerostomia by 2 weeks that may play an important role in helping the patient improve nutrition after treatment and improve his performance score.
Mucositis has been universally identified as the most dose compromising acute side effect associated with head and neck radiotherapy.26 The anticipated incidence of grade ¾ mucositis 69% is with conventional chemoradiotherapy versus 89% in the accelerated radiotherapy protocols with a p-value of 0.0001.15,26,27 In the current study, treatment was well tolerated in both arms. The only observed difference was in the pattern of onset and resolution of the grade 3 mucositis. The study arm did tend to show a shorter duration of grade 3 mucositis by 3 weeks. However, with the exception of one patient in the control arm, no patients needed the requirement of Ryle's tube support at the end of treatment. There were also no treatment breaks on account of mucositis. The other factor related to mucositis that is of concern is dysphagia and its impact on nutrition during treatment. As anticipated the accelerated protocol did evidence an earlier onset of dysphagia by 2 weeks over the control arm. However, surprisingly it was the conventional protocol that was associated with the persistence of grade 3 dysphagia that was statistically significant (p-value = 0.002).
Fatigue is a new parameter that is being considered as a significant toxicity when assessing concurrent chemoradiation protocols in LAHNC. The pivotal study of “SMART-SIB” had observed a surprising increase in fatigue in the conventional arm compared with the “SMART” arm (66.6 vs. 40%) and was statistically significant with a p-value of 0.038.15 However, the authors did not offer a scientific rationale for the same. Newer studies are evaluating the correlation of brainstem especially the medullary doses toward the continuation of fatigue. Ferris et al established a multidimensional fatigue inventory index score as a predictor for the rate of fatigue. As our numbers were too limited to show a significant association, this parameter was not calculated.28 However, the protocol cohort had significantly less observed fatigue, 11 patients (78.5%) versus 15 patients (100%) in the conventional treatment arm. This was also resolved earlier by 3 weeks. Both the lower incidence of fatigue and the shorter duration of fatigue were statistically significant (p-value = 0.005).
The limitations of this study include a small sample size and short follow-up to evaluate the sustained clinical response and late toxicities. The trial endpoints could be further validated by the inclusion of radiobiological parameters in future studies. The key points observed were that patients could tolerate the intensified dose of the study protocol with no increase in parameters of toxicity. The “SMART” technique was equally efficacious in cytoreduction. Although the onset of toxicities like xerostomia, mucositis, and dysphagia was accelerated in the study arm, it did not significantly contribute toward the tolerance of treatment. The shorter duration of dysphagia and mucositis observed in the study arm may be favorable for a faster recovery of nutritional status after treatment.
Conflict of Interest
None declared.
References
- Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 100 randomized trials and 19,248 patients, on behalf of MACH-NC group. Ann Oncol. 2016;27
- [CrossRef] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(06):394-424.
- [Google Scholar]
- First results of a phase III multicenter randomized controlled trial of intensity modulated (IMRT) versus conventional radiotherapy (RT) in head and neck cancer (PARSPORT: ISRCTN48243537; CRUK/03/005) J Clin Oncol. 2009;27:LBA6006. (18S):
- [Google Scholar]
- Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(01):4-14. http://www.sciencedirect.com/science/article/pii/S0167814009001881 Accessed2009
- [Google Scholar]
- Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69:S112-S114. (2, Suppl):
- [Google Scholar]
- Competing causes of death in patients with locoregionally advanced head and neck cancer treated with concomitant boost radiation plus concurrent weekly cisplatin. Clin Transl Oncol. 2013;15(04):321-326.
- [Google Scholar]
- Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(02):127-136.
- [Google Scholar]
- IMRT for head and neck cancer: reducing xerostomia and dysphagia. J Radiat Res (Tokyo). 2016;57:i69-i75. (1, Suppl 1):
- [Google Scholar]
- The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27(02):131-146.
- [Google Scholar]
- Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5(07):516-525.
- [Google Scholar]
- Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2(01)
- [CrossRef] [Google Scholar]
- Tumour repopulation during treatment for head and neck cancer: clinical evidence, mechanisms and minimizing strategies In: InHead and Neck Cancer 2012 Mar 14. IntechOpen;
- [Google Scholar]
- Simultaneous modulated accelerated radiation therapy in the treatment of nasopharyngeal cancer: a local center’s experience. Int J Radiat Oncol Biol Phys. 2006;66(04):40-46.
- [Google Scholar]
- Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45(01):21-32.
- [Google Scholar]
- Randomized controlled study comparing simultaneous modulated accelerated radiotherapy versus simultaneous integrated boost intensity modulated radiotherapy in the treatment of locally advanced head and neck cancer. J Egypt Natl Canc Inst. 2018;30(03):107-115. http://www.sciencedirect.com/science/article/pii/S1110036218300402 Accessed2018
- [Google Scholar]
- Target volume selection and delineation (T and N) for primary radiation treatment of oral cavity, oropharyngeal, hypopharyngeal and laryngeal squamous cell carcinoma. Oral Oncol. 2018;87:131-137.
- [Google Scholar]
- Selection of lymph node target volumes for definitive head and neck radiation therapy: a 2019 Update. Radiother Oncol. 2019;134:1-9. https://www.sciencedirect.com/science/article/pii/S0167814019300234 . Accessed March 4, 2022
- [Google Scholar]
- Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother Oncol. 2011;101(03):394-402.
- [Google Scholar]
- CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(01):83-90.
- [Google Scholar]
- P0107 Dose to dysphagia aspiration-related structures and its effect on swallowing: comparison of 3D CRT and IMRT plans. Eur J Cancer. 2014;50:e38-e39.
- [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) v4.0 Based Hybrid Patient and Physician questionnaire for Head and Neck (HN) Radiotherapy Symptom Reporting. International Journal of Radiation Oncology, Biology, Physics. 2011;81(02):S673. 2011 Oct 1
- [Google Scholar]
- Parotid gland shrinkage during IMRT predicts the time to Xerostomia resolution. Radiat Oncol. 2015;10:19.
- [CrossRef] [Google Scholar]
- Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: a retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol. 2008;47(08):1513-1518.
- [Google Scholar]
- Radical radiotherapy with concurrent weekly cisplatin in loco-regionally advanced squamous cell carcinoma of the head and neck: a single-institution experience. Head Neck Oncol. 2009;1(01):17.
- [CrossRef] [Google Scholar]
- Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002;94(04):1131-1141.
- [Google Scholar]
- Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(02):145-153.
- [Google Scholar]
- Brainstem dose is associated with patient-reported acute fatigue in head and neck cancer radiation therapy. Radiother Oncol. 2018;126(01):100-106.
- [Google Scholar]







