Translate this page into:
Prevalence and clinical significance of FGFR3 genomic alterations in Asian bladder urothelial carcinoma: A systematic review
Corresponding author: Dr. Chandra Sekhar Pedamallu, PhD, FRSB, Department of Bioinformatics, Excelra, Iseline, New Jersey, United States. pcs.murali@gmail.com/chandrasekhar.p@excelra.com
-
Received: ,
Accepted: ,
How to cite this article: Pedamallu R, Ojesina AI, Pedamallu CS. Prevalence and clinical significance of FGFR3 genomic alterations in Asian bladder urothelial carcinoma: A systematic review. Asian J Oncol. 2025;11:1. doi: 10.25259/ASJO_70_2024
Abstract
Bladder urothelial carcinoma is a common malignancy with distinct genetic variations across populations. Fibroblast Growth Factor Receptor 3 (FGFR3) mutations and structural variants play a critical role in its pathogenesis and represent important therapeutic targets. This systematic review was conducted using the repositories such as large genomic data repositories (including cBioPortal and GENIE) and PubMed (from inception to July 2024) to assess FGFR3 alterations in Asian patients. This review reveals at least 14.6% (average of 18.4%) of patients harbored FGFR3 genomic alterations (mutations or structural variants), with the S249C mutation being the most prevalent. Notably, these alterations were primarily mutually exclusive with TP53 mutations. The findings emphasize the clinical importance of FGFR3-targeted therapies, such as the U.S. Food and Drug Administration(FDA)-approved pan-FGFR inhibitor erdafitinib and underscore the necessity of molecular profiling to enhance treatment outcomes in bladder urothelial carcinoma.
Keywords
Bladder urothelial carcinoma
Asian patients
Fibroblast growth factor receptor 3
INTRODUCTION
Bladder urothelial carcinoma is one of the most prevalent malignancies worldwide, characterized by significant variability in incidence and genetic alterations across different populations.[1-4] In 2022, the World Cancer Research Fund reported that over 614,298 new cases of bladder cancer were diagnosed globally. Among these:
-
India: 22,548 cases
-
Japan: 34,568 cases
-
China: 92,883 cases
These statistics highlight the global impact of bladder cancer and the varying incidence rates across different regions. Recently, the Fibroblast growth factor receptor 3 (FGFR3) gene has been identified as a key contributor to the pathogenesis of bladder urothelial carcinoma.[5,6] Mutations and structural variants of FGFR3, particularly in hotspot regions, are increasingly recognized for their oncogenic potential and as promising therapeutic targets.[5,6]
The incidence and genetic landscape of bladder urothelial carcinoma can vary significantly among Asian populations, making the prevalence and implications of FGFR3 alterations particularly noteworthy in this group.[7] Understanding the frequency and nature of these alterations in Asian patients is crucial for developing effective treatment strategies and improving clinical outcomes.[8]
This systematic review study focuses on analyzing the prevalence of FGFR3 mutations and structural variants in bladder urothelial carcinoma among Asian patients. By conducting a systematic review of genomic data, the research aims to elucidate the significance of these alterations and their potential as targets for FGFR3-directed therapies[9], such as the FDA-approved pan-FGFR inhibitor erdafitinib. The findings of this study are expected to enhance the growing body of evidence supporting the use of precision medicine in the treatment of bladder urothelial carcinoma.
MATERIAL AND METHODS
This systematic review was conducted using the following repositories:
-
1.
Large genomic data repositories, including cBioPortal and American Association for Cancer Research (AACR) Project Genomics Evidence Neoplasia Information Exchange (GENIE)
-
2.
PubMed, from inception to July 2024
The inclusion criteria combined the keywords “Bladder Urothelial Carcinoma,” “Bladder Cancer,” “Asian,” “Primary,” and “Genomic.” From the large-scale genomic repositories, we identified three relevant datasets out of 11 after applying the inclusion criteria and removing duplicates.
From the PubMed search, 132 records were identified after applying the inclusion criteria. Several of these records overlap with the three datasets identified in large-scale genomic repositories, with only one additional relevant record found. Table 1 summarizes the studies on primary bladder urothelial carcinoma used to determine the prevalence of FGFR3 mutations in the Asian population. The datasets from large-scale genomic repositories were retrieved and analyzed using cBioPortal[10-12] and GENIE Cohort v16.0,[13] both open-access cancer genomics databases. The additional dataset from PubMed was retrieved from Peak et al.[14] The analysis focused on genomic alterations, including mutations, copy number variants, and structural variations/fusions.
| Dataset ID | Studies | Ethnicity | No. of Asian patients of the total number of patients in the dataset |
|---|---|---|---|
| Bladder_Dataset1 | TCGA Bladder urothelial carcinoma[1,15] | Asian | 43 of 411 patients (10.46%) |
| Bladder_Dataset2 | Bladder urothelial carcinoma[16] | Asian-far east/Indian subcontinent |
35 of 1,014 patients (3.45%) |
| Bladder_Dataset3 | AACR Project genomics evidence neoplasia information exchange (GENIE) cohort v16.0 public (https://genie.cbioportal.org/)[13] | Asian |
104 of 2,845 patients (3.65%) |
| Bladder_Dataset4 | Urothelial carcinoma of the bladder[14] | Asian |
279 of 8,728 patients (3.19%) |
TCGA: The cancer genome atlas, AACR: American association for cancer research
RESULTS
Table 2 presents the mutation and structural variants of FGFR3 identified in the primary bladder urothelial carcinoma studies analyzed. Figure 1 illustrates the FGFR3 gene alterations in the three selected datasets of Asian bladder urothelial carcinoma patients. FGFR3 is the most frequently altered gene in Asian bladder urothelial carcinoma patients.[7] FGFR3 alterations predominantly include mutations and structural variants.
| Dataset_ID | FGFR3 mutation in Asian patients | FGFR3 Structural variants/fusions in Asian patients | FGFR3 Copy number variants in Asian patients | Total FGFR3 genomic alterations in Asian patients | Total FGFR3 genomic alterations in Asian patients |
|---|---|---|---|---|---|
| Bladder_Dataset1 |
28% (12/43) |
14% (6/43) |
9% (4/43) |
44% (19*/43) |
19% (77/411) |
| Bladder_Dataset2 |
20% (7/35) |
3% (1/35) |
0% (0/35) |
23% (8/35) |
28% (280/1014) |
| Bladder_Dataset3 |
14% (15/104) |
2% (2/104) |
0% (0/104) |
16% (17/104) |
24% (671/2845) |
| Bladder_Dataset4 |
14.6% (41/279 |
NA | NA |
14.6% (41/279) |
18.3% (1605/8728) |
| FGFR3 genomics alterations (in summary) |
18.4% (85/461) |
20.3% (2633/12998) |
|||
NA – Data not available, FGFR3: Fibroblast growth factor receptor 3.
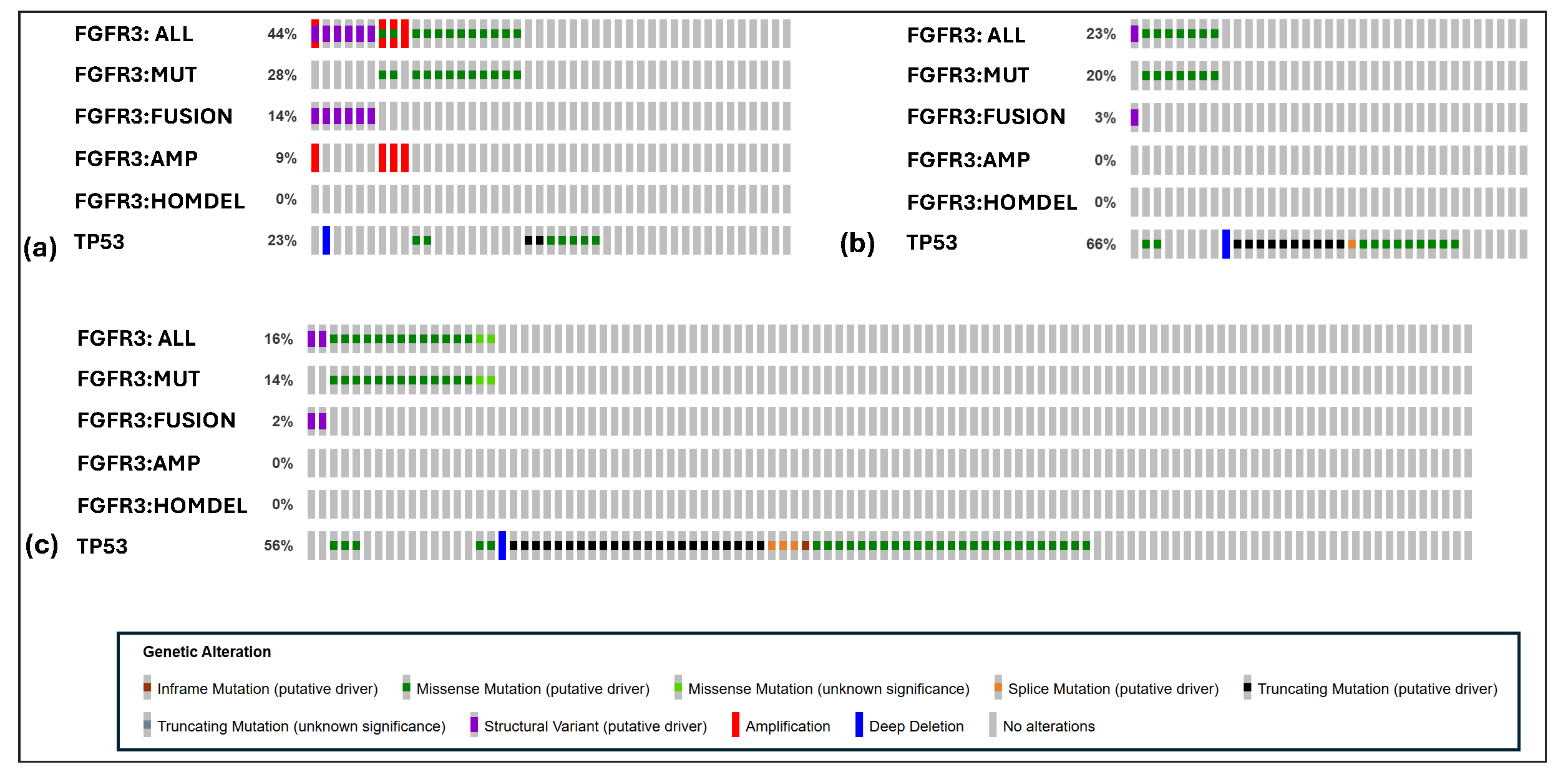
- FGFR3 genomic alterations assessed in Asian patients. This figure was generated using cBioPortal web tools. (a) FGFR3 alterations in Bladder_Dataset1 (b) FGFR3 alterations in Bladder_Dataset2(c) FGFR3 alterations in Bladder_Dataset3
Figure 2 illustrates landscape of FGFR3 mutations in the three selected datasets of Asian bladder urothelial carcinoma patients. The TCGA bladder urothelial carcinoma study reports a few patients with FGFR3 copy number amplifications; however, these amplifications co-occur with mutations or structural events. Notably, mutations and structural events are mutually exclusive FGFR3 mutations, frequently identified in bladder urothelial carcinoma studies, are predominantly driver mutations and account for a significant portion of oncogenic events. In addition, the FGFR3-TACC3 fusion is recognized for its strong oncogenic potential. FGFR3 genomic alterations are observed in at least 14.6% (average of 18.4%) of Asian patients. The overall frequency of FGFR3 alterations (including mutations, copy number variations, and structural variants in all ethnicities) is 19%, 28%, 24%, and 18.3% in Bladder_dataset1 to Bladder_dataset4, respectively.
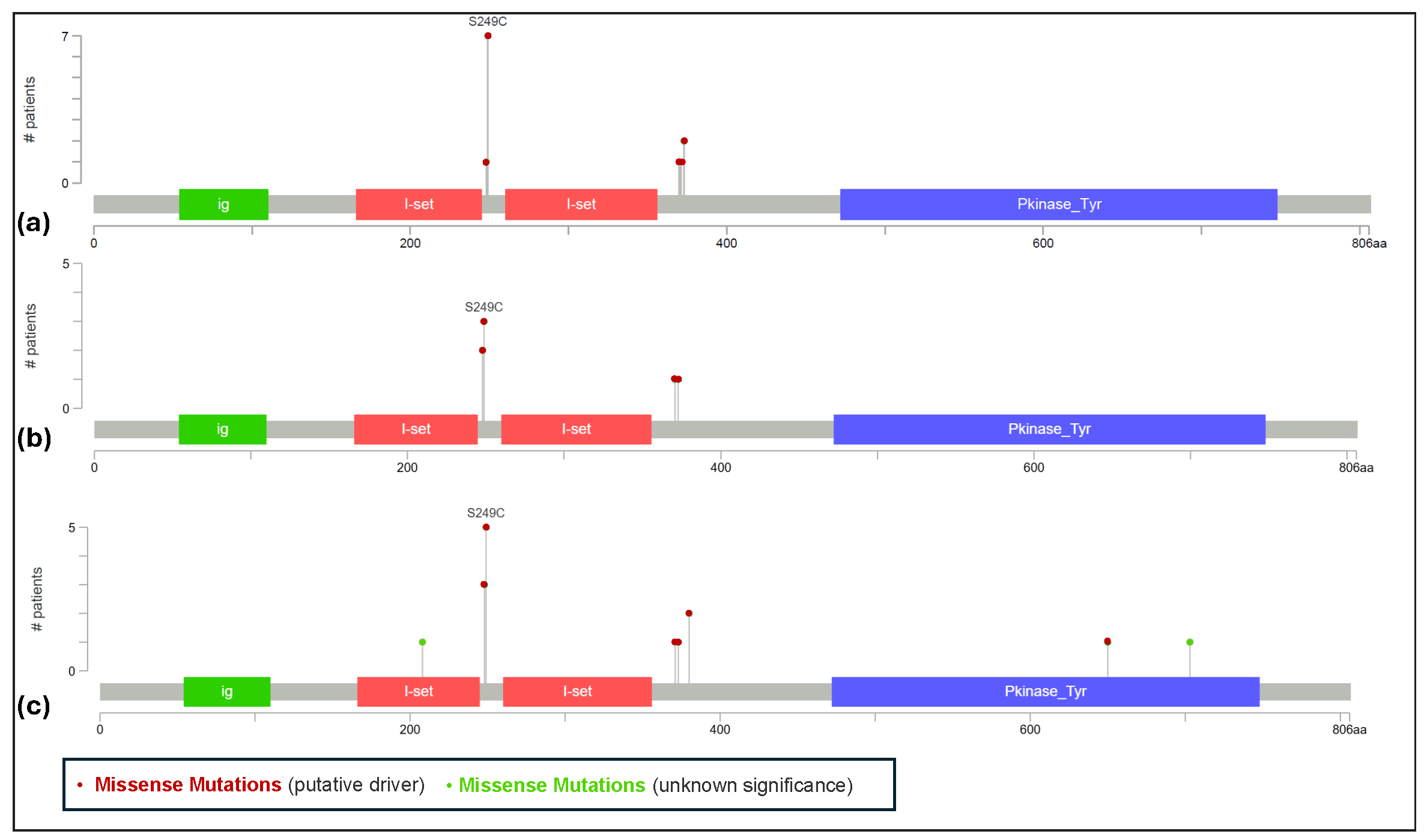
- Landscape of FGFR3 mutations in bladder urothelial carcinoma patients. This figure is generated using cBioPortal web tools. (a) Lollipop schemes for FGFR3 in Bladder_Dataset1(b) Lollipop schemes for FGFR3 in Bladder_Dataset2 (c) Lollipop schemes for FGFR3 in Bladder_Dataset3
Table 3 highlights the prevalence of FGFR3 genomic alterations across different ethnic groups in Bladder_Dataset1, revealing statistically significant differences:
-
Between Asian vs. White patients: p = 0.0001 (Fisher’s exact test).
-
Between Asian vs. African American patients: p = 0.0047 (Fisher’s exact test).
| Dataset_ID | Ethnicity comparison |
Fischer’s exact test P-value (FGFR 3 genomic alteration frequencies between ethnicities) |
|---|---|---|
| Bladder_Dataset1 | Asian (vs) White |
0.0001 (19/43 vs 53/327) |
| Asian (vs) African American |
0.0047 (19/43 vs 2/23) |
|
| Bladder_Dataset2 | Asian-Far East/Indian Subcontinent (vs) White |
0.5677 (8/35 vs 245/849) |
| Asian-Far East/Indian Subcontinent (vs) African American |
1.0 (8/35 vs 8/38) |
|
| Bladder_Dataset3 | Asian (vs) White |
0.0614 (17/104 vs 578/2374) |
| Asian (vs) African American |
0.3674 (17/104 vs 21/95) |
|
| Bladder_Dataset4 | Asian (vs) European genomic ancestry |
0.1332 (41/279 vs 1368/7447) |
FGFR3: Fibroblast growth factor receptor 3
In contrast, no statistically significant differences were observed in other datasets, indicating that these findings may be specific to Bladder_Dataset1 or influenced by datasetspecific factors.
Table 4 presents the prevalence of FGFR3 genomic alterations alongside other recurrent molecular alterations, including TP53, KMT2D, KDM6A, ARID1A, PIK3CA, KMT2C, RB1, EP300, E2F3, PPARG, MDM2, and CDKN2A.[17] In addition to FGFR3 genomic alterations, TP53 and CDKN2A exhibit the highest frequency of genomic alterations.
| Frequently altered genes |
Bladder_Dataset1 (n = 43) |
Bladder_Dataset2 (n = 35) |
Bladder_Dataset3 (n=104) |
Bladder_Dataset4 (n=279) |
|---|---|---|---|---|
| FGFR3 | 44% | 23% | 16% | 14.6% |
| TP53 | 23% | 66% | 56% | 61% |
| KMT2D | 21% | 20% | 30% | NA |
| KDM6A | 28% | 31% | 30% | NA |
| ARID1A | 12% | 26% | 25% | 22% |
| PIK3CA | 12% | 14% | 17% | 14% |
| KMT2C | 23% | 26% | 27% | NA |
| RB1 | 14% | 34% | 25% | 18% |
| EP300 | 9% | 14% | 13% | NA |
| E2F3 | 9% | 11% | 15% | NA |
| PPARG | 9% | 7% | 7% | NA |
| MDM2 | 12% | 14% | 10% | 9% |
| CDKN2A | 35% | 14% | 15% | 39% |
n=size of the patient population
NA: Data not available, FGFR3: Fibroblast growth factor receptor 3
DISCUSSION
FGFR3 mutations, frequently identified in bladder urothelial carcinoma studies, are predominantly driver mutations and account for a significant portion of oncogenic events. In addition, the FGFR3-TACC3 fusion is recognized for its strong oncogenic potential. Notably, mutations in FGFR3 and TP53 are typically mutually exclusive across all datasets.
Current treatment strategies in Asian patients vary depending on the stage of the cancer. Different treatment modalities for the treatment of bladder cancer include intravesical instillation of Bacillus calmette-Guerin (BCG), transurethral resection of bladder tumor radical cystectomy with urinary diversion, immunotherapy, neoadjuvant chemotherapy and chemoradiation. Given that a subset of bladder cancer patients harbors FGFR3 mutations, it is crucial to identify these alterations prior to initiating treatment, especially in Asian populations. Several FGFR inhibitors have been developed in recent years for various cancers, including bladder cancer. FGFR inhibitors such as infigratinib and futibatinib (used in cholangiocarcinoma), and pazopanib (used in renal cell carcinoma) are among the therapies that target FGFR pathways.
In 2019, The U.S. FDA granted accelerated approval to Balversa (erdafitinib) for treating adult patients with locally advanced or metastatic bladder cancer that has FGFR3 or FGFR2 genetic alterations.[17] Common side effects include increased phosphate levels, dry skin, anemia, and eye problems. Balversa also requires further clinical trials to confirm its benefits and is used alongside an FDA-approved diagnostic tool such as the therascreen Fibroblast growth factor receptor rotor-gene Q real-time reverse transcription polymerase chain reaction (FGFR RGQ RT PCR) Kit.[17] Balversa is the first personalized treatment targeting these specific genetic mutations. In the Janssen Pharmaceutical clinical trial, patients selected in the trail had progressed following treatment with chemotherapy. In the same clinical trial, it achieved a 32.2% overall response rate (with 2.3% having a complete response and almost 30% having a partial response), with responses lasting about 5.5 months.[18] This highlights the critical role of identifying these genomic alterations in the management and treatment of bladder urothelial carcinoma.
CONCLUSION
This study highlights the significant prevalence of FGFR3 mutations and structural variants in bladder urothelial carcinoma among Asian patients. The systematic review revealed that at least 16% of the patients exhibited FGFR3 alterations, with the S249C mutation being the most frequent hotspot. These findings underscore the importance of molecular profiling in managing bladder cancer and identifying actionable mutations for targeted therapy.
The clinical relevance is further emphasized by the FDA-approved pan-FGFR inhibitor erdafitinib, which targets FGFR3 mutations and recurrent fusions. The mutual exclusivity observed between mutations and structural events suggests distinct oncogenic pathways, informing future therapeutic approaches. Continued research and development of FGFR-targeted therapies could significantly improve outcomes for bladder urothelial carcinoma patients, especially within the Asian demographic.
Author contributions
RP: Conceptualization, formal analysis, methodology, reviewing, writing and editing; CSP: Visualization, data curation, supervision, reviewing, writing and editing; AO: Methodology, writing, reviewing and editing.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
REFERENCES
- Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The global burden of urinary bladder cancer: An update. World J Urol. 2020;38:1895-904.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bladder cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. [accessed 2024 Sep 01]. https://www.ncbi.nlm.nih.gov/books/NBK536923/
- [Google Scholar]
- The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J Glob Health. 2023;13:04109.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The roles of FGFR3 and c-MYC in urothelial bladder cancer. Discov Oncol. 2024;15:295.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of FGFR3 in bladder cancer: Treatment landscape and future challenges. Cancer Treat Rev. 2023;115:102530.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring racial disparities in bladder urothelial cancer: insights into survival and genetic variations. African Journal of Urology. 2024;30:28. [accessed 2024 Aug 01]. https://doi.org/10.1186/s12301-024-00430-5
- [CrossRef] [Google Scholar]
- Prognostic markers in invasive bladder cancer: FGFR3 mutation status versus P53 and KI-67 expression: a multi-center, multi-laboratory analysis in 1058 radical cystectomy patients. Urol Oncol. 2022;40:110.e1-110.e9.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted therapies: Expanding the role of FGFR3 inhibition in urothelial carcinoma. Urol Oncol. 2022;40:25-36.
- [CrossRef] [PubMed] [Google Scholar]
- The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- [accessed 2024 May]. Available online: https://www.cbioportal.org/
- AACR project GENIE: Powering precision medicine through an international consortium. Cancer Discov. 2017;7:818-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative genomic landscape of urothelial carcinoma of the bladder among patients of East and South Asian genomic ancestry. Oncologist. 2023;28:e910-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genomic heterogeneity as a barrier to precision oncology in urothelial cancer. Cell Rep. 2022;41:111859.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genomic heterogeneity as a barrier to precision oncology in urothelial cancer. Cell Rep. 2022;41:111859.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- FDA approves first targeted therapy for metastatic bladder cancer. FDA News Release; 2019. [accessed 2024 Sep 06]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-metastatic-bladder-cancer







