Translate this page into:
Minimal residual disease evaluation by flow cytometry in B cell acute lymphoblastic leukemia (BCP-ALL) and its outcome in children and adults
*Corresponding author: Dr. Biren Parikh MD,DCP, Department of Oncopathology, Gujarat Cancer and Research Institute, Chamanpura, Ahmedabad, India. birenparikh2002@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nagarjun B, Parikh B, Vora H, Raiya B. Minimal residual disease evaluation by flow cytometry in B cell acute lymphoblastic leukemia (BCP-ALL) and its outcome in children and adults. Asian J Oncol. 2025;11:4. doi: 10.25259/ASJO-2023-17-(489)
Abstract
Objectives
Acute lymphoblastic leukemia (ALL) is a most common childhood malignancy. It is a heterogeneous group of diseases with variable responses to therapy. Multiple risk factors have been identified to predict the risk of relapse and chance of cure. None of the risk factors could independently predict the relapse risk. Measurable residual disease (MRD) has emerged as an independent powerful prognostic indicator and tailor treatment accordingly to achieve higher cure rate. To evaluate the MRD status in children and adult population and analyse the outcomes between the two groups.
Material and Methods
A prospective study was conducted in 138 patients diagnosed with B-cell phenotype ALL (BCP-ALL). This study included all the diagnosed cases of BCP-ALL patients in whom MRD was assessed. Bone marrow examination was simultaneously performed.
Results
MRD positive was observed in 24% (33/138) and was negative in 76% (105/138). 5 year event free survival in MRD positive was 44.1% and 87.5% in MRD negative. Risk of disease recurrence was significantly higher in high risk group. The 5 year event free survival (EFS) based on MRD negative, MRD 0.01 to 0.1%, > 0.1 to 1% and greater than 1% was 87.5%, 41.7%, 71.4% and 0% respectively.
Conclusion
This is one of the few detailed reports from India in which the EFS of both adults and children treated with same protocol was studied. Study highlights the significant association of MRD status with EFS. MRD levels helps in deciding better informed clinical decisions, management and prognosis.
Keywords
Adults
B-all
Children
Flow cytometry
MRD
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. It comprises of a heterogeneous group of diseases with variable responses to therapy. Multiple risk factors have been identified to predict the risk of relapse and chance of cure.[1] The risk factors include the sex of patient, age at diagnosis, and disease characteristics such as eg, white blood cell count at diagnosis, immunophenotype, and structural and numeric chromosomal aberrations.
Despite remarkable advances in treatment of ALL, the long-term remission rate in children is nearly 80%. Whereas in adults the remission rates range between 15-40%.[1,2] None of the above said risk factors could independently determine or predict response to therapy. In search of an independent prognostic and predictive variable, measurement of in vivo treatment effectiveness was studied and proved to be a promising tool.[3-9]
In older treatment protocols, response to therapy was conventionally assessed by microscopic examination, but this is quite challenging when the leukemic cell population is less. Especially in ALL, the blast cell morphology is often indistinguishable from hematogones, generally seen after chemotherapy and transplantation. Due to these limitations morphologic remission could sometimes be imprecise.[10] The residual disease is identified by the persistence of leukemic blasts at levels below the conventional morphologic detection limit and is defined as minimal/measurable residual disease (MRD), which is detected by flow cytometry or molecular diagnostic support.[11]
MRD has emerged as a powerful prognostic indicator in childhood ALL as it helps identify the risk of relapse. Several protocols presently stratify risk based on MRD and tailor the treatment accordingly, with the goal of achieving higher cure rates with minimum toxicity.[12,13] Various methods, such as quantification of clonal rearrangements of immunoglobulins, PCR-based assays, and multi-flow cytometry (MFC) are employed.
MRD by MFC is increasingly used in the management of ALL due to its high applicability, sensitivity, and specificity. It is an independent prognostic finding, which is a better determinant than classical risk factors.[14]
This study tried to evaluate the MRD status of children and adults undergoing treatment for B-cell phenotype ALL (BCP-ALL), emphasizing outcomes between the two groups.
MATERIAL AND METHODS
A prospective study was conducted in patients diagnosed with BCP-ALL at a Regional Cancer Center in Gujarat from 1 November 2019 to 31 May 2022. It included all the diagnosed cases of BCP-ALL patients in whom MRD was assessed post-induction. The diagnosis of BCP-ALL was based on morphology, cytochemistry, and immunophenotypic antigen expression as per the WHO 2016 criteria.
Bone marrow (BM) examination was simultaneously done with MRD assessment. The first pull was sent for MRD assessment, and the remaining sample was put onto a glass slide for preparation of a blood film using slides. The bone marrow films were then air-fixed and stained by the May-Grunwald-Giemsa stain.
BM samples were processed for eight-color MFC immunophenotyping using the bulk lyse and stain method. The cell suspension was prepared by bulk erythrocyte lysing with an ammonium chloride-based lysing reagent. After the lysis and wash step, 100 µL of the sample was taken in 2 tubes, and suitable antibodies were added to each. After 10 minutes of incubation, the samples were centrifuged with sheath fluid; the pellet was resuspended in sheath fluid. Samples were acquired on a three-laser BD Bioscience Fluorescence activated cell sorting (BD FACS) instrument with FACS diva acquisition software. The number of events collected per tube ranged from 750,000 to 1,000,000.
MRD analysis was performed using the 8-color two-tube antibody panel. Samples were labeled MRD positive based on the identification of a cluster of a minimum of 10 events with at least two leukemia-associated immunophenotype (LAIP) or any difference from normal approach (DfN). The markers in tubes were: tube 1: CD45, CD19, CD34, CD10, CD20, CD58, CD66, and CD38 Tube 2: CD45, CD19, CD34, CD10, CD73, CD81, CD123 and CD200.
An initial analysis gate was drawn around the leucocyte population based on CD19/SSC properties. A further gate was used to define CD19 positive events derived from the total leucocyte gate. The gates were created using both CD45 and CD10 against markers of interest. CD19, CD45, CD10, and CD34 are backbone markers as they are necessary for basic gating for separating B cells (normal and abnormal) and identifying normal maturation. MRD is reported positive based on defined criteria of MRD level when it is > 0.01% and negative when < 0.01%.
Statistical analysis
Statistical analysis was performed with statistical package for social science (SPSS) software version 28 (IBM SPSS Statistics for Windows, Armonk, New York, United States; IBM Corp). Event-free survival (EFS) curves were estimated by the Kaplan-Meier method and compared according to the log-rank test. The starting point for the observation time was the date of diagnosis. Relapses were considered events in the calculation of EFS probability.
RESULTS
We analyzed 138 samples of BCP - ALL patients treated with BFM-90 between 2017 and 2022; the baseline patient population and MRD characteristics are described in Table 1. There were 85 males, accounting for 62% of the patient population. The patients were stratified into three different subsets depending on age. The number of patients in each subset was 51.8% (71/138), 30% (41/138), and 18.2% (26/138) in the age groups of 1-9 years (subset -1), 10 -19 years (subset - 2), and greater than 20 years (subset - 3), respectively. The mean leukocyte count at baseline was 19.05×103, 50.53×103, and 36.45×103 in the subsets 1, 2, and 3, respectively. Cytogenetic karyotyping was available in 64/138 patients [Table 2].
| Parameter (n = 138) | MRD positive (n =33) (%) | MRD negative (n=105) (%) |
|---|---|---|
| Age | ||
| 1-9 (71/138) (subset -1) | 15/33 (45.4%) | 56/105 (53.3%) |
| 10–19 (41/138) (subset -2) | 10/33 (30.3%) | 31/105 (29.5%) |
| >20 (26/138) (subset -3) | 8/33 (24.2%) | 18/105 (17.1%) |
| Sex | ||
| Male (85/138) | 23/33 (69.6%) | 62/105 (59.0%) |
| Female (53/138) | 10/33 (30.4%) | 43/105 (40.9%) |
| WBC count baseline | ||
| Missing (14/138) | 06/14 | 08/14 |
| <50,000 (106/124) | 24/27 (88.8%) | 82/97 (84.5%) |
| >50,000 (18/124) | 3/27 (11.1%) | 15/97 (15.4%) |
| Cytogenetics (64) | ||
| Missing (74) | 18/74 | 56/74 |
| High risk (13/64) | 6/15 (40%) | 07/49 (14.2%) |
| Not high risk (51/64) | 9/15 (60%) | 42/49 (85.7%) |
| Risk category | ||
| SR (100/138) | 12/33 (36.3%) | 88/105 (83.8%) |
| IR (09/138) | 06/33 (18.18%) | 03/105 (2.8%) |
| HR (29/138) | 15/33 (45.4%) | 14/105 (13.3%) |
MRD: Measurable residual disease, WBC: White blood cell, SR: Standard risk, IR: Intermediate risk, HR: High risk
| Age | Normal karyotyping (NK) | t (9:22) | Others | MRD positivity |
|---|---|---|---|---|
| 01-09 years | 19 | 01 | t (12:21) - 02 | 05 – NK, 01 – t (9:22) |
| 10-19 years | 14 | 04 | t (1:19) and MLL rearrangement – 01 each | 02 – NK, 02 – t (9:22), MLL rearrangement - 01 |
| Above 19 years | 15 | 07 | None | 02 – NK, 02 – t (9:22) |
NK: Normal karyotying, MRD: Minimal residual disease, MLL: Mixed lineage leukemia gene
MRD assessment was done post-induction day 33 in patients who attained a morphological CR. MRD was evaluated among all the BCP-ALL patients who achieved a morphological CR and reported positive based on defined criteria of MRD level > 0.01% and negative when < 0.01%. MRD was positive in 24% (33/138) and negative in 76% (105/138) of patients. Among those who tested positive, 12 (8.7%) had MRD levels between 0.01% and 0.1%, 14 (10.1%) fell in the MRD range between 0.1% and 1%, and a level > 1% was observed in 7 (5.0%) patients. Of the 33 patients with positive MRD, cytogenetics were available for 13 and were as follows: seven patients had normal karyotyping, five with t (9:22), and one had MLL rearrangement.
Patients were stratified into standard risk (SR), intermediate risk (IR), and high risk (HR) as per the pre-defined characteristics in the BFM -90 protocol. Among the evaluated 138 patients, 100 (72.5%) fell into SR, whereas IR and HR had nine (6.5%) and 29 (21%) patients, respectively.
MRD positivity assessed as per the risk categories, was observed to be 13 (13%), six (66.7%), and 15(51.7%) in SR, IR, and HR, respectively. An event was considered when patients were lost to follow-up, relapse, or death and were censored. The number of events in SR, IR, and HR were 14 (14%), 04 (44.4%), and 15 (51.7%), respectively. The median EFS was 70, 41, and 16 months.
Various factors such as age, sex, baseline White blood cell (WBC) count, and risk category were assessed for the presence of statistical significance with the MRD status and the result was as follows:
Relapses during or after treatment were considered an event. The median follow-up was 20.0 months (1.0 -71.0 months). The 2-year EFS for MRD negative and positive patients was 84% and 46%, respectively. Nineteen (57.6%) out of 33 MRD positive and 13 (12.3%) out of 105 MRD negative had an event. The median EFS in the MRD positive group was 17 months, and that in the MRD negative group was not reached [Figure 1]. The difference in the EFS was statistically significant in the MRD positive and negative groups (p < 0.001 by log rank test). The median EFS based on the level of MRD negative, MRD 0.01 to 0.1%, 0.1 to 1%, and > 1% were not reached by 26 months, 15 months, and 8 months, respectively.

- Median event free survival in MRD positive population was 17 months and in MRD negative population it was not achieved. (MRD: Minimal residual disease, EFS: Event free survival)
As per age, the study population was divided into 3 subsets: 1 to 9 years (subset -1), 10 to 19 years (subset - 2), and ≥ 20 years (subset - 3). The number of events in each subset 1, 2, and 3 were 11, 13, and 9, respectively, and the estimated median EFS in each subset was 41 months, 16 months, and 16 months, respectively, in MRD positive patients. The MRD negative patients’ EFS was not reached [Figure 2]. This difference in EFS was statistically significant (p < 0.01).
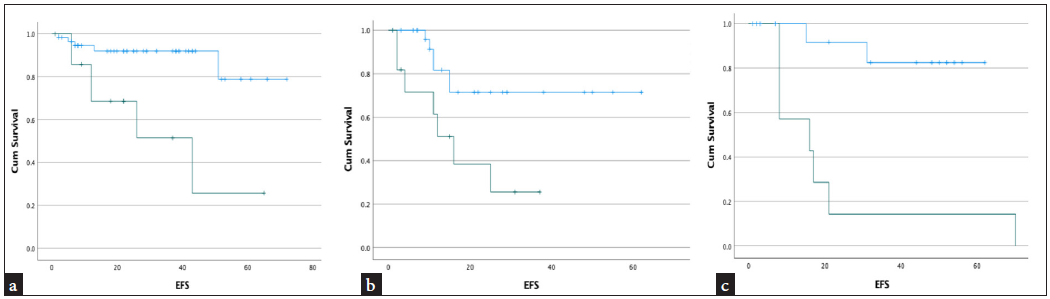
- Kaplein Meyer plot showing median event free survival (EFS) in different study population with MRD positive. (a): Population aged 1-9 years median EFS was 41 months, (b): Study group of 10-19 years age, EFS was 16 months, (c): Patients 20 years and above, EFS was 16 months. In all the study population with MRD negative median EFS was not reached. (blue - MRD negative population, Green - MRD positive population, EFS: event free survival)
DISCUSSION
Flow cytometry based MRD assessment is an easy and cost-effective method. It has a rapid turnaround time.[15] MRD is not routinely practiced in developing nations like India. However, with number of studies advocating for its significance in therapeutic decisions, the number is expected to increase. Presently centres with available facilities have incorporated MRD assessment in routine practice to determine the prognosis.[16-18] A positive MRD is a strong predictor of relapse in patients diagnosed with ALL.[19-21] Patients with positive MRD have an increased risk of relapse with conventional chemotherapy, hence dose escalation is recommended in some studies. For patients with negative MRD status on day 8 of induction, the chemotherapy dose can be de-escalated to avoid long-term complications of chemotherapy.[22]
Detection of MRD is not only important in newly diagnosed ALL patients but also has its significance in relapsed ALL cases and in patients with isolated extramedullary relapse.[23] Patients with a negative MRD, when taken up for hematopoietic stem cell transplantation, performed better as compared to the relapsed patients who showed a positive MRD after re-induction.[24,25] There is very little data on MRD and its predictive and prognostic utility in developing countries like India. In this retrospective study, we collected data of 138 BCP-ALL patients who underwent MRD testing at our institute.
The sex ratio in our studies was similar to other studies quoted in the Indian and western literature with a male preponderance.[26,27] Thirty-three out of 138 (24%) patients showed a positive MRD post-induction day 33. Borowitz et al. reported MRD positivity of 19.4%. MRD positivity is quite variable ranging from 25-60% as these studies have included both B and T cell acute lymphoblastic leukemia (T-ALL).[28,29] MRD positivity was not significantly related to sex, leukocyte count, high risk cytogenetics, or national cancer institute (NCI) risk category in our study and this is consistent with other studies.[30,31]
Although there are multiple published reports on the use of flow based MRD from India, not many have correlated MRD with outcome. This study tried to access the relationship between age groups like 1-9 years (subset - 1), 10 -19 years (subset - 2), and > 19 years (subset-3) with MRD positivity rate and their effect on the disease-free survival. This is the first study to assess such a relation in India. The event-free survival in MRD positive patients in subset-1 was 41 months, whereas the MRD positive patients in subset-2 & 3 had a similar EFS of 16 months.
The limitation of our study was that the treatment was not risk-adapted, therefore, patients with MRD positivity did not receive treatment escalation due to practical issues. This intervention could have had a better impact on the outcomes. Longer follow-up is needed for adequate events in the MRD negative group to estimate the EFS in different age subsets. This report on both children and adults treated with a uniform protocol is one of the few detailed reports from India. We could show a significant difference in the EFS in BCP- ALL patients showing a positive and a negative MRD. Patients with a positive MRD had poor EFS. Children aged < 10 years with MRD positivity had better EFS. MRD levels help in better-informed clinical decisions, management and prognosis.
CONCLUSION
MRD levels helps to recognize treatment efficacy, identify relapse before it is morphologically overt which helps in better informed clinical decisions, management and prognosis.
Author contribution
BRN: Manuscript preparation and data collection; BP: Proof reading; HV: MRD analysis and statistical analysis; BR: MRD analysis and inputs.
Ethical approval
The research/study approved by the Institutional Ethics Committee at Gujarat Cancer and Research Institute, number IRC/2023/P-94, dated 20th September 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Molecular response to treatment redefines all prognostic factors in children and adolescents with b-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206-14.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol. 2010;150:389-405.
- [CrossRef] [PubMed] [Google Scholar]
- Chemotherapy in 998 unselected childhood acute lymphoblastic leukaemia patients: Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122-33.
- [PubMed] [Google Scholar]
- Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94:1209-17.
- [PubMed] [Google Scholar]
- Risk and response-based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the pediatric oncology group (POG) and children’s cancer group (CCG) Blood. 2007;109:926-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early response to therapy and outcome in childhood acute lymphoblastic leukemia: A review. Cancer. 1997;80:1717-26.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal residual disease (MRD) analysis in the non MRD-based ALLIC-BFM 2002 Protocol for childhood ALL: Is it possible to avoid MRD testing? Leukemia. 2008;22:989-97.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the interfant-99 protocol. Leukemia. 2009;23:1073-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of minimal residual disease monitoring in adult and pediatric acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:1083-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Monitoring minimal residual disease in acute leukemia: Technical challenges and interpretive complexities. Blood Rev. 2017;31:63-75.
- [CrossRef] [PubMed] [Google Scholar]
- Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: Multicentric assessment is feasible. Cytometry B Clin Cytom. 2008;74:331-40.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical relevance of detection of minimal residual disease in childhood acute lymphoblastic leukemia. J Clin Pathol. 2003;56:249-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic significance of monitoring leukemia-associated immunophenotypes by eight-color flow cytometry in adult b-acute lymphoblastic leukemia. Blood Cancer J. 2013;3:e133.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Post-induction measurable residual disease using multicolor flow cytometry is strongly predictive of inferior clinical outcome in the real-Life management of childhood t-Cell acute lymphoblastic leukemia: A study of 256 patients. Front Oncol. 2020;10:577.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of minimal residual disease identifies differences in treatment response between t-ALL and precursor b-ALL. Blood. 2002;99:4386-93.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346-55.
- [CrossRef] [PubMed] [Google Scholar]
- Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31:2736-42.
- [CrossRef] [PubMed] [Google Scholar]
- Slower molecular response to treatment predicts poor outcome in patients with TEL/AML1 positive acute lymphoblastic leukemia: Prospective real-time quantitative reverse transcriptase-polymerase chain reaction study. Cancer. 2003;97:105-13.
- [CrossRef] [PubMed] [Google Scholar]
- Rearrangement status of the malignant cell determines type of secondary igH rearrangement (V-replacement or V to DJ joining) in childhood B precursor acute lymphoblastic leukemia. Leukemia. 1997;11:1258-65.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal residual disease after intensive induction therapy in childhood acute lymphoblastic leukemia predicts outcome. Leukemia. 1998;12:675-81.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2013;14:199-209.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7-12.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal residual disease analysis for the prediction of relapse in children with standard-risk acute lymphoblastic leukaemia. Br J Haematol. 1998;100:235-44.
- [CrossRef] [PubMed] [Google Scholar]
- The significance of graft-versus-host disease and pretransplantation minimal residual disease status to outcome after allogeneic stem cell transplantation in patients with acute lymphoblastic leukemia. Blood. 2001;98:1982-4.
- [CrossRef] [PubMed] [Google Scholar]
- Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477-89.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and outcome of B-cell acute lymphoblastic leukemia in patients older than 9 years: A single center experience of 241 cases from AIIMS, New Delhi, India. J Clin Oncol. 2013;31(15-suppl):7082.
- [CrossRef] [Google Scholar]
- Children’s Oncology Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children’s Oncology Group study. Blood. 2008;111:5477-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Flow cytometry based MRD and its impact on survival outcome in children and young adults with ALL: A prospective study from a tertiary cancer centre in southern india. Indian J Hematol Blood Transfus. 2020;36:300-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002;100:52-8.
- [CrossRef] [PubMed] [Google Scholar]
- Flow cytometry evaluation of minimal residual disease in acute lymphoblastic leukaemia type B. Open Leuk J. 2010;3:47-54.
- [Google Scholar]








