Translate this page into:
Lymph Node Involvement in T1a Glottic Carcinoma: Controversial Case Series
Address for correspondence Eda Tuna Yalcinozan, MD, Department of Otorhinolaryngology, Faculty of Medicine, Near East University, Yakın Dogu Blv., Nicosia 99138, Cyprus. dr.etuna@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The treatment modality of laryngeal cancer is complete resection of the primary lesion and dissection of the cervical lymph nodes that is related to their staging. Following the patient without neck dissection is the usual expectation in T1 glottic carcinomas. However, a combination of elective neck dissection with surgical treatment for clinically N0 neck in early glottic tumors may be an effective treatment modality in the prevention of missed micrometastases. In these two case reports, elective neck dissection and re-examination of the treatment were discussed accordingly. We aimed to point out the importance of evaluation, examination, and, if it is uncertain, moreover the dissection of lymph nodes in early-stage glottic carcinoma since there are rarely any studies available.
Keywords
glottic carcinoma
lymph node
T1a
Introduction
Cervical lymph node metastasis is one of the most important determinants in the prognosis of the head and neck squamous cell carcinomas (SCCs), which reduces the likelihood of local control and survival. However, in the treatment modality of laryngeal cancer, complete resection of the primary lesion and dissection of the cervical lymph nodes are the essential principle. Lymph node dissection is still controversial in the clinically N0 neck. Since the risk of lymph node metastasis is particularly low in T1 to 2 glottic cancers, neck dissections are not routinely performed in those cases. In these two case reports, elective neck dissection and re-examination of the treatment were discussed accordingly.
Case Report 1
A 50-year-old female patient who had an intermittent voice disorder for 2 to 3 months appeared in our clinic as her complaint did not regress for the past 1 month. On physical examination, direct laryngoscopy revealed a polypoid tissue at the anterior two-third of the left vocal cord with the hyperemic smooth surface and it was ∼5 mm in size, but there was no pathological finding detected by inspection and palpation in neck examination. According to these findings, a direct laryngoscopic biopsy was performed under a microscopic view (Fig. 1A). The pathological report was presented as laryngeal SCC. Afterward, the neck computerized tomography (CT) of the patient showed oval reactive lymph nodes with a short axis of 6 mm on level II, short axis of 4 mm on level III and IV, and short axis of 5 mm on level V on both sides, that did not form a group. Minimal asymmetry was seen on the left vocal cord that protrudes into the anterior lumen and obliteration appearance at the inferior of the left piriform sinus was found.

-
Fig. 1 (A) Left vocal cord preoperative view. (B) Preoperative magnetic resonance imaging findings: left vocal cord mass in size of 8 x 4 mm (white arrow).
In her magnetic resonance imaging (MRI), the left piriform sinus was asymmetric in the anterior of the left vocal cord, and an 8 × 4 mm sized, well-contoured T1 isointense, T2 hyperintense homogeneously enhancing lesion that did not expand to the surrounding tissue was found (Fig. 1B). The patient underwent frontolateral hemilaryngectomy and level II to III superior selective neck dissection. In the left jugulodigastric region, approximately two lymph nodes were found and also nine lymph nodes were present in level II and four lymph nodes were present in level III. During the operation, the surgical margin was determined by the frozen section, and according to this, neither the posterior commissure nor the anterior commissure was infiltrated with SCC.
Histopathological examination results were reported as moderately differentiated, glottic laryngeal SCC with the involvement of level II lymph node (T1aN1M0). Because of the jugulodigastric lymph node metastasis, she was consulted to the oncology on the same day. Oncology department decided to initiate the radiotherapy treatment onto the neck and primary tumor site after 2 months later. Radiotherapy was performed with an average of 6540 rad intensity-modulated radiation therapy technique in the presence of second-line cisplatin chemotherapy. The patient is followed up without local and neck recurrence in the postoperative third year.
Case Report 2
A 65-year-old male patient presented with a severe voice annoyance since ∼4 to 5 years. On physical examination, direct laryngoscopy revealed a posteriorly leukoplasia, generally hyperemic broad-based polypoid tissue of ∼10 × 5 mm at the posterior one-third of the left vocal cord, which was not clearly associated with the posterior commissure and vocal cords were mobile. In the neck, lymphadenopathy was detected at the jugulodigastric region, ∼10 × 10 mm in size. Ultrasonography (USG) revealed a suspicious appearance of lymphadenopathy in the jugulodigastric region at a size of 15 × 6 mm.
The patient underwent direct laryngoscopic biopsy under microscopic view. The pathological specimen reported as a laryngeal SCC. In his MRI findings, there was a lesion with enhancement on the middle one-third portion of the left vocal cord (Fig. 2A) and a jugulodigastric lymph node in the left cervical region in size of 10 × 15 mm (Fig. 2B). Laryngofissure cordectomy and lateral selective neck dissection were performed in accordance with this information. Histopathological examination was reported as less differentiated glottic laryngeal SCC and absence of nodal metastasis (T1aN0M0). There was no local or neck recurrence at 2nd year postoperatively.
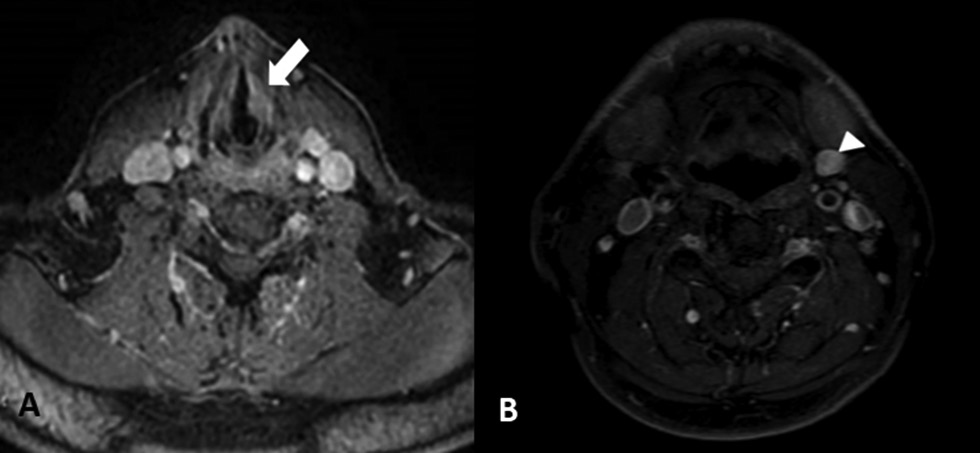
-
Fig. 2 (A) Preoperative magnetic resonance imaging (MRI) findings, mass lesion on the middle one-third portion of the left vocal cord (white arrow). (B) Preoperative MRI findings (white arrowhead): a jugulodigastric lymph node in the left cervical region in size of 10 x 15 mm.
Discussion
It is necessary to treat the neck in patients with lymph node metastasis; therefore, we can wait and see in clinically N0 neck. Even so elective surgical treatment modality instead of waiting in the clinically N0 neck can also be done. In a study conducted by Mnejja et al suggested that in glottic or supraglottic T1N0M0 and T2N0M0 tumors, superselective (levels IIa and III) neck dissection can limit morbidity without increasing the rate of failure, and therefore can be performed in patients without palpable cervical lymphadenopathy. They emphasized that T3 and T4 tumors require functional neck dissection as occult lymph node metastasis is more frequent.1 In cases with N0 laryngeal cancer if any cervical lymph node metastasis is suspected, unilateral or bilateral elective neck dissection can be performed according to the location of the tumor. Postoperative radiotherapy must be applied in cases where histopathological (+) metastasis is detected in the pathological specimens. However, to put a certain concern, the number of glottic cancers was very low in this study as well the others.2
A total of 38 head and neck cancer patients (16 oropharynx, 2 hypopharynx, 8 preoral cavities, and 12 larynx cancers) were evaluated and clinically diagnosed as N0 with USG and CT. In sentinel lymph node, biopsies that were taken intraoperatively were detected as positive in five patients including two glottic larynx carcinoma.3 In a study by van den Brekel et al, it was revealed that USG-guided fine-needle aspiration biopsy (US-FNAC) has high specificity in the detection of clinically occult metastases. They indicated that US-FNAC is a very sensitive method in N0 necks and also lowers the wrong determination chance.4 These studies also highlighted the importance of histopathological diagnosis in staging the tumor and in determining the treatment to be performed thereafter, along with the clinical evaluation of lymph nodes. In a study conducted by Elô et al, 206 patients with clinically N0 neck were evaluated. Of these, 61 were diagnosed with T1 glottic cancer and treated with endolaryngeal laser method. Neck follow-up was partially performed with direct inspection and palpation or with frozen section. Follow-up only is preferred for T1a glottic laryngeal carcinoma patients with clinically (–) lymph node. N0 neck dissection has been performed and among 61 patients in the 2-year follow-up, only 1 patient (1.5%) had significant metastases. Independent from the other factors, complete endoscopic examination and neck follow-up were considered as sufficient because the metastasis rates of vocal cord T1a cancers are very low.5
The studies related to nodal metastasis distribution in clinically and radiologically negative necks showed that levels IIB and IV were the nodes that wouldn’t be involved in the first line without metastasis of other nodes. Thus, neck-level IIA and III dissections may be sufficient in elective surgery of supraglottic and glottic cancers.6 In parallel to this study, in 2012, Chone et al showed that none of the patients with clinically N0 had metastasis at level IV. In the histopathological examination of patients with clinically positive lymph nodes 25% showed involvement at level IV, and metastases with levels II and III involvement in every case were found. However, this study was performed in T2 to T4 glottic and supraglottic carcinoma patients, not in the T1 glottic tumors.7
Conclusion
The combination of elective neck dissection with surgical treatment for clinically N0 neck in early glottic tumors may be an effective treatment modality in the prevention of missed micrometastases and future recurrences. However, there are little or even no studies in the literature, with only early stage T1a to T1b glottic tumors and/or early stage glottic tumor cases. For this reason, it is necessary to investigate the results of elective neck dissection treatment in early-stage glottic tumors with larger series of cases.
Conflict of İnterest
None declared.
References
- Occult lymph node metastasis in laryngeal squamous cell carcinoma: therapeutic and prognostic impact. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(05):173-176.
- [Google Scholar]
- The relationship between the localization, size, stage and histopathology of the primary laryngeal tumor with neck metastasis. Eurasian J Med. 2014;46(01):1-7.
- [Google Scholar]
- Value of sentinel lymphonodectomy in head and neck cancer patients without evidence of lymphogenic metastatic disease. Auris Nasus Larynx. 2001;28(04):339-344.
- [Google Scholar]
- Considerations in the treatment of the node-negative (N0) neck in glottic carcinomas. Pathol Oncol Res. 2002;8(04):257-261.
- [Google Scholar]
- Selective Neck Dissection (2A, 3): A rational replacement for complete functional neck dissection in patients with N0 supraglottic and glottis carcinoma. Laryngoscope. 2008;118:4.
- [Google Scholar]
- Levels II and III neck dissection for larynx cancer with N0 neck. Rev Bras Otorrinolaringol (Engl Ed). 2012;78(05):59-63.
- [Google Scholar]







