Translate this page into:
Insulinoma presenting as complex partial seizures
Address for correspondence: Dr. Brahmanandam Lingudu, Department of Endocrinology, Andhra Medical College, King George Hospital, Visakhapatnam - 530 002, Andhra Pradesh, India. brahmaendo2k2@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We describe a 42-year-old male patient who presented with recurrent left focal seizures with altered sensorium refractory to polytherapy with antiepileptic drugs and recurrent hypoglycemic episodes. Whipple's triad was documented. Insulin, C-peptide levels were elevated, and insulin-glucose ratio was more than 0.3 confirming the endogenous hyperinsulinism. Magnetic resonance imaging (MRI) abdomen revealed mass in the tail and distal body of the pancreas. Interictal electroencephalography showed generalized slow wave activity in delta range. MRI brain was normal. Excision of tumor was done. Histopathology confirmed the insulinoma. The patient had no recurrence of seizures during follow-up.
Keywords
Electroencephalography
endogenous hyperinsulinism
hypoglycemia
seizures
Introduction
Insulinoma is a very rare pancreatic neuroendocrine tumor with an incidence about one in 250,000 patient years. Insulinomas may occur in either sex at any age. They are twice as common in women than men with fifty percentage of the subjects are over fifty. Approximately, 80% of insulinomas are due to solitary adenomas which average about 2 cm in diameter, 10% are due to multiple adenomas, and 5% are associated with multiple endocrine neoplasia 1 (MEN 1). Neuroglycopenic symptoms such as conscious disorder, abnormal behavior, psychiatric symptoms or seizures were observed in cases of insulinoma.1 Hence, insulinoma is frequently misdiagnosed as neurological disorder or psychiatric disorder. Insulinomas may also present as transient neurological deficits, confusion, amnesia, personality change, lethargy, decreased motor activity, gradual decline in cognition, anxiety, palpitations, tremor, weakness, and visual disturbances such as diplopia, blurring of vision.2 Because of the presence of both neurological and psychiatric symptoms sometimes insulinomas may also present as neuropsychiatric disorder. Insulinoma may rarely present as complex partial seizures as in our case study. Besides complex partial seizures, it can also present as generalized tonic–clonic seizures, myoclonic seizures, simple partial seizures, refractory seizures, and juvenile myoclonic epilepsy as per the literature.
Case Report
A 42-year-old male patient presented with recurrent attacks of major hypoglycemic episodes of 5 years’ duration. Each episode was characterized by excessive sweating, paresthesias, altered sensorium, generalized weakness requiring third personnel assistance. These episodes occurred between 4 am and 5 am and were abolished with oral or intravenous glucose. There was a history of focal seizures involving left upper limb associated with altered sensorium of 2 years duration. He was started on sodium valproate and later clobazam and oxcarbazepine were added with no response. The patient was referred to endocrine unit for further evaluation. The patient had left focal seizures with impaired consciousness on the 2nd day of hospital admission at 6.45 am with a corresponding blood sugar of 31 mg/dL. Seizures subsided and consciousness regained after giving intravenous 25% dextrose. On questioning, there was a history of excessive weight gain, and he had hypertension recently. He was not a known diabetic and not on any drugs which can cause hypoglycemia. He had no history suggestive of pituitary hormonal excess or hyperparathyroidism. Physical examination and neurological examination was normal.
Table 1 shows baseline biochemical profile. Whipple's triad was documented with a low plasma glucose of 20 mg/dl. The corresponding insulin and C-peptide levels were elevated, and insulin-glucose ratio was more than 0.3 confirming endogenous hyperinsulinism. Imaging was done to localize the lesion. Ultrasound abdomen and contrast enhanced computer tomography (CECT) abdomen were normal. Contrast-enhanced magnetic resonance imaging (CEMRI) abdomen showed 3 cm × 3 cm × 2.7 cm exophytic mass in the tail and distal body of pancreas suggestive of pancreatic neuroendocrine tumor Figure 1. Electroencephalography (EEG) during interictal period showed generalized slow wave activity symmetrically in delta range sometimes predominantly in the frontal region lasting from 2 to 10 s intermittently during the record. Photic stimulation and hyperventilation did not show any additional abnormality. There was no epileptiform discharges in the record. MRI brain was normal. As the patient has pancreatic neuroendocrine tumor, we evaluated for MEN 1. No evidence of either anterior pituitary hormone excess or hyperparathyroidism was reported. Table 2 shows hormonal profile. MRI Pituitary was normal.
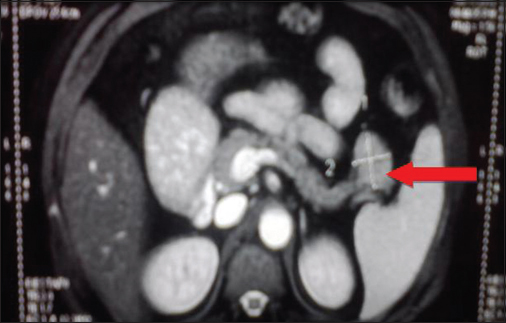
- Contrast-enhanced magnetic resonance imaging abdomen: Well defined round to oval exophytic mass lesion of size 3 cm × 3 cm × 2.7 cm arising from tail and distal body of pancreas suggesting the possibility of pancreatic neuroendocrine tumor
|
Parameter |
Result |
|---|---|
|
Hb% |
12.8 g% |
|
TC |
9800 |
|
DC |
P 66%, L 36%, E 4% |
|
ESR |
18 mm/h |
|
Serum creatinine |
1.4 mg% |
|
Blood urea |
29 mg% |
|
Serum sodium |
142 mEq/L |
|
Serum potassium |
4.6 mEq/L |
|
Serum chloride |
101 mEq/L |
|
Total bilirubin |
0.7 mg/dL |
|
Direct bilirubin |
0.2 mg/dL |
|
SGPT |
30 U/L |
|
SGOT |
38 U/L |
|
Alkaline phosphatase |
88 U/L |
|
Serum calcium |
9.0 mg/dL |
|
Serum phosphorous |
3.1 mg/dL |
|
Serum albumin |
5.1 mg/dL |
P - Polymorphs; L - Lymphocytes; E - Eosinophils; Hb% - Hemoglobin; TC - Total leucocyte count; DC - Differential leucocyte count; ESR - Erythrocyte sedimentation rate; SGPT - Serum glutamate pyruvate transaminase; SGOT - Serum glutamate oxaloacetate transaminase
|
Parameter |
Result |
|---|---|
|
Total T3 |
1.41 ng/mL |
|
Total T4 |
7.14 µg/dL |
|
TSH |
2.87 µIU/mL |
|
Serum prolactin |
27.68 ng/mL |
|
Serum cortisol |
18.93 µg/dL |
|
FSH |
7.25 µIU/mL |
|
LH |
6.43 µIU/mL |
|
Serum testosterone |
462 ng/dL |
|
Basal GH |
0.08 ng/mL |
|
1 h after glucose GH |
<0.05 ng/mL |
|
2 h after glucose GH |
<0.05 ng/mL |
|
Serum PTH |
59.71 pg/mL |
T3 - Tri-iodothyronin; T4 - Thyroxine; TSH - Thyroid stimulating hormone; FSH - Follicular stimulating hormone; LH - Leutinizing hormone; GH - Growth hormone; PTH - Parathyroid hormone
The patient underwent laparotomy and enucleation of tumor. He had transient hyperglycemia following surgery which subsided with diet and exercise in 4 weeks. Antiepileptic drugs (AEDs) were stopped postoperatively, and he never had a recurrence of focal seizures or hypoglycemic episodes during 2 years of follow-up. Table 3 shows trends in fasting plasma glucose, fasting insulin, fasting C-peptide levels. Repeat EEG was normal at the end of follow-up. Histopathology Figure 2 and immunohistochemistry confirmed the diagnosis of insulinoma. Immunohistochemistry showed positive staining for synaptophysin, chromogranin, neuron-specific enolase and negative for CK7 and CK20 suggesting pancreatic neuroendocrine tumor. Ki 67 staining showed proliferative index of 10% suggesting benign nature of the tumor.
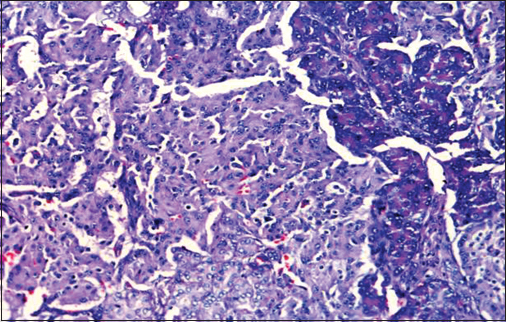
- Histopathological examination of insulinoma: Shows sheets of uniform round cells with moderate cytoplasm and arranged in a trabecular pattern. Nuclei showing granular chromatin. Intervening stroma is fibrovascular and shows congested sinusoidal vascular spaces. Mitotic activity is minimal <10/10 HPF
|
At the time of diagnosis |
2 weeks after surgery |
2 years after surgery |
|
|---|---|---|---|
|
Fasting plasma glucose (mg/dL) |
20 |
125 |
91 |
|
Fasting plasma insulin (µU/mL) |
122.4 |
47.02 |
26.24 |
|
Fasting C-peptide (ng/mL) |
16.23 |
10.65 |
3.89 |
Discussion
We report a rare case of insulinoma who presented with complex partial seizures refractory to polytherapy with AEDs. The clinical presentation of insulinoma is usually insidious. The interval from presentation to diagnosis ranged from 1 month to 30 years.3 The delay in diagnosis is mainly due to three factors. First, the symptoms of insulinoma lack specificity, including various seizure disorders, personality change, amnesia, bizarre behavior and incidentally dystonia and polyneuropathy. These symptoms are similar to many neurological and psychiatric disorders. Second, fasting plasma glucose levels may be normal in some patients. Third, hypoglycemia itself may decrease counterregulatory hormonal response to hypoglycemia and induce unawareness of autonomic and neuroglycopenic symptoms.4 In our case, the delay was 5 years and probably due to hypoglycemic unawareness and complex partial seizures.
EEG during hypoglycemia usually shows diffuse or focal slowing and enhanced response to hyperventilation5 and this represents hypometabolism of brain. Interictal EEG in our patient showed generalized slow wave activity in delta range. Epileptiform discharges in hypoglycemia may be due to desynchronization between neuronal excitation and inhibition due to neuroglycopenic damage.6 Experimental studies confirmed that severe hypoglycemia induces spontaneous synchronous discharges in vitro and in vivo, thus generate hypermetabolic state and further deplete the reserve energy of brain. These seizures cannot be blocked by common AED. Hypoglycemic seizures induced in experimental animals tend to originate in mesial temporal lobe structures such as amygdala and hippocampus, which have a low threshold for seizures. These findings are helpful to explain the refractory nature of hypoglycemic seizures in insulinoma and complex partial seizures of temporal origin. In our case, the seizures were refractory to AED. Two earlier case studies by Wang et al.7 and Graves et al.8 reported refractory complex partial seizures secondary to insulinoma.
Insulinomas are usually <2 cm in size, and conventional CT scan is not beneficial.9 Endoscopic sonography and MRI are useful to localize small pancreatic insulinomas10 In our patient, ultrasound abdomen and CECT abdomen did not localize the lesion, and CEMRI abdomen showed a mass in tail and distal body of pancreas suggesting probably MRI can be better option for localization of small pancreatic insulinomas wherever endoscopic sonography is unavailable. Treatment of choice for insulinoma is surgical excision. It may be simple enucleation of tumor for small and benign adenomas or distal pancreatectomy if localized in tail and distal body of the pancreas. Whipple's procedure or total pancreatectomy is needed for larger, multiple, and malignant tumors. In our case, an exophytic mass was located in tail and distal body of the pancreas and enucleation was done, and no recurrence was observed during follow-up.
Conclusion
We report a case of insulinoma presenting with refractory complex partial seizures. To the best of our knowledge, worldwide this was the third case report of insulinoma presenting as complex partial seizures with eventual recovery.
Financial support and sponsorship
Nil.
Acknowledgments
We would like to thank Department of Radiology and Department of Pathology of Andhra Medical College, King George Hospital, Visakhapatnam for their valuable support.
Conflicts of interest
There are no conflicts of interest.
References
- Neuroglycopenic and other symptoms in patients with insulinomas. Am J Med. 1999;106:307-10.
- [Google Scholar]
- Functioning insulinoma – Incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin Proc. 1991;66:711-9.
- [Google Scholar]
- Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med. 1993;329:834-9.
- [Google Scholar]
- The effect of hypoglycemia on the electroencephalogram at varying degrees of oxygenation of the blood. Am J Physiol. 1942;136:1-6.
- [Google Scholar]
- Regionally selective metabolic effects of hypoglycemia in brain. J Neurochem. 1981;36:1952-8.
- [Google Scholar]
- An insulinoma with clinical and electroencephalographic features resembling complex partial seizures. J Zhejiang Univ Sci B. 2008;9:496-9.
- [Google Scholar]
- Misdiagnosis of seizures: Insulinoma presenting as adult-onset seizure disorder. J Neurol Neurosurg Psychiatry. 2004;75:1091-2.
- [Google Scholar]
- Endoscopic ultrasound in the localisation of pancreatic islet cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:177-93.
- [Google Scholar]
- Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195-211.
- [Google Scholar]







