Translate this page into:
Evaluation of High-Risk Human Papillomavirus Testing in Women with Atypical Squamous Cells of Undetermined Significance to Detect Cervical Intraepithelial Neoplasia
Address for correspondence Shunji Suzuki, MD, Department of Obstetrics and Gynecology, Japanese Red Cross Katsushika Maternity Hospital, 5-11-12 Tateishi, Katsushika-ku, Tokyo, Japan. czg83542@mopera.ne.jp
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction We retrospectively examined the changes in the management and outcomes of the cases with atypical squamous cells of undetermined significance (ASC-US) according to the Bethesda system (TBS).
Materials and Methods Between May 2009 and December 2018, 432 cases with ASC-US were retrospectively examined for the implementation of high-risk human papillomavirus (hrHPV) testing and/or cervical biopsy and clinical courses.
Results The hrHPV testing was performed in 277 cases (64.1%). Of these, there were 80 with positive hrHPV (28.9%). Of the 80 cases, cervical biopsy was performed in 71 (88.9%) cases based on the finding of colposcopy. Cervical intraepithelial neoplasia (CIN) was diagnosed in 55 cases (77.4%). The sensitivity, specificity, positive predictive value, and negative predictive value of CIN diagnosis by hrHPV testing in cases of ASC-US were 72.5, 95.0, 88.7, and 86.3%, respectively (p < 0.01).
Conclusion The conducting of hrHPV test on women who were evaluated as ASC-US according to TBS affirmed its clinical usefulness.
Keywords
atypical squamous cells of undetermined significance
the Bethesda system
high-risk human papillomavirus testing
clinical usefulness
cervical intraepithelial neoplasia
Introduction
The Bethesda system (TBS) is a system for reporting cervical or vaginal cytologic diagnoses used for reporting Pap smear results.1 It was introduced in 1988 and revised in 1991 to 2014.2,3,4 By 1994, almost 90% of laboratories in the United States were using TBS. This quick acceptance of TBS has been thought to be due to as follows: (1) nomenclature that provides uniform diagnostic terminology; (2) diagnostic categories that incorporate the latest scientific information on the pathogenesis and prognosis of cervical lesions; and (3) incorporation of the evaluation of specimen adequacy as an integral part of the report.2 TBS for reporting cervical cytology has been now a “must have” for pathologists, cytopathologists, pathology residents, cytotechnologists, and clinicians.1,2,3,4
In addition, in TBS 2014 high-risk human papillomavirus (hrHPV) testing was strongly recommended as the most cost-effective triage test for cases of atypical squamous cells of undetermined significance (ASC-US), which is the most common cytological abnormality reported on Pap tests leading to colposcopic follow-up and/or treatment of the women.4
In 2007 in Japan, the multiple academic societies considered whether to adopt TBS, and in 2008 the Japan Association of Obstetricians and Gynecologists (JAOG) recommended TBS adopted nationwide.5 For example, the Guideline for Gynecological Practice in Japan 2020 recommends as follows; when cytology screening results indicate ASC-US, use HPV testing to determine the need for colposcopy and cervical biopsy.6 In addition, since 2000 the HPV testing in cases of ASC-US has been covered by insurance, and the burden on the patient has been reduced from about $ 200 to $ 10.7 Our institute is one of the major university hospitals in Tokyo, and the evaluation system of cervical cytology has been revised to TBS at the same time as the recommendation by the JAOG. In particular, TBS has been adopted since May 2009.
Based on these backgrounds, we retrospectively examined the changes in the management and outcomes of the cases with ASC-US in our institute.
Materials and Methods
The study was conducted after approval by the Ethics Committee of the Japanese Red Cross Katsushika Maternity Hospital (2008). Informed consent to the retrospective analysis of data was obtained from all subjects.
At our institute, we have performed cervical cytology in all patients with informed consent at the time of their first visits. In the management for the cases with ASC-US, the discretion has been dealt of each gynecologist referring to the gynecological guidelines at that time.
Between May 2009 and December 2018, cervical cytology was performed in 43,210 women, and ASC-US was defined in 1,002 women (2.3%). Of the 1,002 cases, 432 cases (43.1%: maternal age: average 41, range: 16–84 years old) excluding 570 cases during follow-up of abnormal cytology were retrospectively examined for the implementation of HPV testing and/or cervical biopsy and clinical courses.
Statistical Analysis
Data are presented as numbers (%). Statistical analyses were performed by means of the statistical software SAS version 8.02 (SAS Institute, Cary, North Carolina, United States).
Results
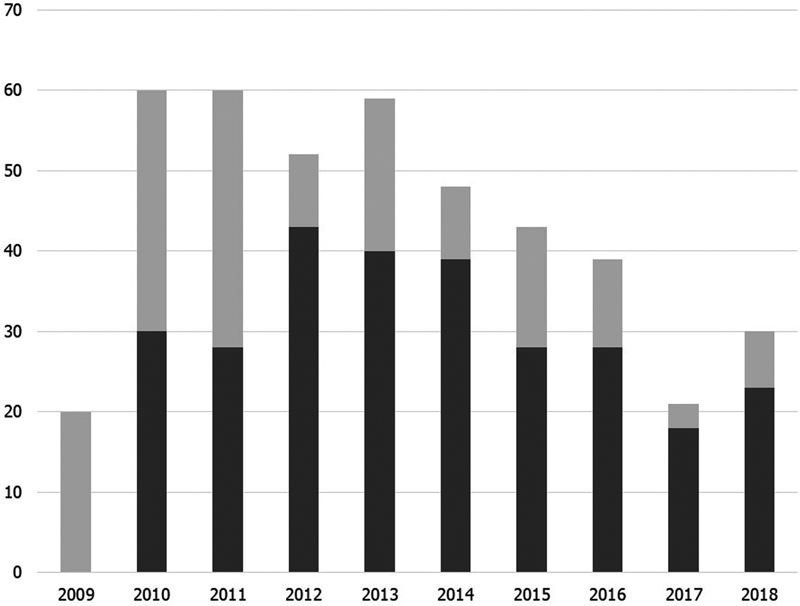
Fig. 1 shows the implementation status of hrHPV testing in cases of ASC-US by year. The implementation rate in 2009 was 0% and in 2011 was 46.7%; however, it increased to 82.7% in 2012 (p < 0.01). Since then, the implementation rate has remained at 65 to 86% without any significant difference. As a whole, the hrHPV testing was performed in 277 cases (64.1%).
-
Fig. 1 Implementation status of high-risk human papillomavirus (hrHPV) testing in cases of atypical squamous cells of undetermined significance by year between May 2009 and December 2018. Dark gray shows the number of cases underwent hrHPV, while light gray shows the number of cases without hrHPV testing.
Of the 277 cases, there were 80 cases with positive hrHPV (28.9%). Of the 80 cases, cervical biopsy was performed in 71 (88.9%) cases based on the finding of colposcopy. Cervical intraepithelial neoplasia (CIN) 1, 2, and 3 were diagnosed in 42 (59.2%), 10 (14.1%), and 3 (4.2%), respectively (total: 55, 77.4%).
Of the 197 cases, 27 (/197, 13.7%) cases with negative hrHPV underwent cervical biopsy at the discretion of the attending gynecologists, and 16 cases were diagnosed as CIN1 (59.3%). One year later, 116 of the remaining 170 cases with negative hrHPV underwent cytology at our institute, and 111 (95.7%) and 5 (4.3%) of them were evaluated as normal (= negative for intraepithelial lesion or malignancy) and ASC-US, respectively. Therefore, CIN1 to 3 was finally diagnosed in 25.6% (71/277) of the total cases who underwent the hrHPV testing. Therefore, the sensitivity, specificity, positive predictive value, and negative predictive value of CIN diagnosis by hrHPV testing in cases of ASC-US were 72.5, 95.0, 88.7, and 86.3%, respectively (p < 0.01).
Of the 155 cases without HPV testing, cervical biopsy was performed in 68 (43.9%) cases based on the findings of colposcopy. CIN 1, 2, and 3 were finally diagnosed in 45 (29.0%), 6 (3.9%), and 2 (1.2%), respectively (total: 53, 34.2% of the whole, p = 0.08 vs. cases underwent hrHVP testing).
Discussion
Based on the current results, the positive and negative predictive values of CIN diagnosis by hrHPV testing in cases of ASC-US were statistically high. In addition, there were no case of CIN3 requiring cervical conization in cases with negative hrHPV. Therefore, the conducting of hrHPV test on women who were evaluated as ASC-US reaffirmed its clinical usefulness concerning the avoiding of unnecessary colposcopy and cervical biopsy. Since 2012, a hrHPV testing has been performed on more than two-thirds of the women with ASC-US. To date, hrHPV testing has been reported to be important in cervical cancer screening for triage to colposcopy.4,8 Proper use of HPV testing has been suggested to improve the management of women with cytologic abnormalities. When used properly, it may reduce morbidity and mortality and do so in a cost-effective manner.9 In addition, some studies have also introduced the hrHPV testing in conjunction with cervical cytology during routine cervical cancer screening.8 The current results may support these previous studies.4,8,9
During the study period, however, there were some women who required colposcopy and cervical biopsy without HPV testing to reduce the number of outpatient visits. The denials of the presence of the virus leading to cervical cancer by a nonintrusive test may have a benefit of giving women the confidence and peace of mind requiring no treatment; however, it is also important for life to reduce the burden of going to the hospital. We believe that the colposcopy test itself does not put an undue burden on the women; however, cervical biopsy will put various burdens on the women such as discomfort and atypical genital bleeding. Fortunately, our colposcopy tests have been able to identify the women requiring cervical biopsy with about the same accuracy as hrHPV testing. The current findings will not show off our diagnostic capability for colposcopy; however, they will indicate that HPV testing can compensate an inadequate diagnostic capability of gynecologists unfamiliar with colposcopy.
Strengths and Limitations
We understand that there are some serious limitations in this study. Although the prevalence of abnormal findings in this study seemed to be reasonable compared with those in some previous studies,10 the sample size of this study may be small. Moreover, in this retrospective study, we cannot verify the presence of diagnosis difference or reliability among physicians.
In addition, discrepancies between abnormal cervical cytology or hrHPV status (cytology negative/HPV positive) and subsequent histological findings are a common occurrence. For the cases, a careful review of the hrHPV status and the degree of cytological atypia have been recommended to be performed before further intervention.11 Although the discrepancies were also observed in the current study, we could not examine them retrospectively in detail. Therefore, a further study may be needed concerning the discrepancies.
Conclusion
Based on the current results, the conducting of hrHPV test on women who were evaluated as ASC-US according to TBS affirmed its clinical usefulness; however, there seems to be room for consideration of revised TBS tailored to the circumstances of women in Japan.
Conflict of Interest
None declared.
Funding None.
References
- The 1988 Bethesda System for reporting cervical/vaginal cytologic diagnoses. Developed and approved at the National Cancer Institute Workshop, Bethesda, Maryland, U.S.A., December 12-13, 1988. Acta Cytol. 1989;33(05):567-574.
- [Google Scholar]
- The Bethesda System 2001: an update of new terminology for gynecologic cytology. Clin Lab Med. 2003;23(03):585-603.
- [Google Scholar]
- The Bethesda System for Reporting Cervical Cytology, Definitions, Criteria, and Explanatory Notes London, UK: Springer; 2015.
- [Google Scholar]
- The Pap Test and Bethesda 2014. “The reports of my demise have been greatly exaggerated.” (after a quotation from Mark Twain) Acta Cytol. 2015;59(02):121-132.
- [Google Scholar]
- Labor and Welfare: Cancer screening guidelines (in Japanese)
- Guideline for Gynecological Practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J Obstet Gynaecol Res. 2021;47(01):5-25.
- [Google Scholar]
- Human papillomavirus genotyping test. Mod Med. 2013;59(11):284-290. . (in Japanese)
- [Google Scholar]
- Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010;118(02):116-122.
- [Google Scholar]
- Human papillomavirus testing in primary cervical screening and abnormal Papanicolaou management. Obstet Gynecol Surv. 2006;61:S15-S25. (6, Suppl 1):
- [Google Scholar]
- Immediate histologic correlation in women with atypical squamous cells of undetermined significance cytology and positive high-risk HPV: a retrospective review of 6000 cases in a large academic women's hospital. Cancer Cytopathol. 2020;128(11):852-859.
- [Google Scholar]
- Cytohistological discrepancies of cervico-vaginal smears and HPV status. Ginekol Pol. 2017;88(05):235-238.
- [Google Scholar]







