Translate this page into:
Epidemiology and outcome of glioblastoma multiforme: A tertiary care experience
*Corresponding author: Dr. Shahid Rashid Sofi, Department of Radiation Oncology, Sheri Kashmir Institute of Medical Sciences, Srinagar, India. rashidshahid853@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tali TA, Amin F, Sofi SR, Sofi MA, Dar NA. Epidemiology and outcome of glioblastoma multiforme: A tertiary care experience. Asian J Oncol, 2023;9:3.
Abstract
Objectives
To study the epidemiology and treatment outcome of glioblastoma multiforme in a tertiary care hospital.
Materials and Methods
This was a retrospective study performed in the Department of Radiation Oncology, Sher-I-Kashmir Institute of Medical Sciences, Soura, Srinagar, India, in which clinical and epidemiological details of the 80 cases diagnosed with glioblastoma multiforme from January 2016 to December 2020 were analyzed.
Results
The majority of the patients in our study were males, compared to females (n = 57, 23: 71%, 29%). Neurodeficiency and headache were the most common presenting symptoms. All patients were subjected to surgery followed by chemoradiation, and the overall median survival was 13 months.
Conclusion
Multimodality therapy, including safe, optimal surgical resection combined with adjuvant radiotherapy or concurrent chemoradiation and sequential chemotherapy, is recommended for all patients with this fatal neoplasm.
Keywords
Glioblastoma Multiforme
Epidemiology
Surgery
Radiotherapy
INTRODUCTION
Glioblastoma (GBM) is the most aggressive diffuse glioma of astrocytic lineage and is considered a grade IV glioma based on the WHO classification.[1] GBM is the most common malignant primary brain tumor, making up 54% of all gliomas and 16% of all primary brain tumors.[2] GBM remains an incurable tumor with a median survival of only 15 months.[3] Treatment is complex, initially consisting of maximally safe surgical resection followed by radiation therapy (RT) and concurrent Temozolomide (TMZ) chemotherapy.[4] GBM is primarily diagnosed at an older age with a median age of 64 at diagnosis.[2,5] The incidence increases with age, peaking at 75–84 years and dropping after 85 years.[2] GBM is uncommon in children.[2] DNA methylation patterns for pediatric and adult groups are similar, but there are distinct clusters that are predominantly found in children and adolescents. GBM is most commonly located in the supratentorial region (frontal, temporal parietal, and occipital lobes), with the highest incidence in the frontal lobe, multiple lobes (overlapping tumors), followed by the temporal and parietal lobes.[5] GBM is rarely located in the cerebellum and is very rare in the spinal cord,[6,7] with different tumor behaviors found at these locations.[6] Cerebellar location of GBM is more common in younger patients (50–56 years of age); supratentorial location is prevalent in older patients (62–64 years of age) and cerebellar location is rare (0.4–3.4%) in this age bracket.[8] Cerebellar GBM is less common in Whites and is smaller in size. [7-9] For spinal cord GBMs, the mean age is 27 years, with a male predominance; 53% of these tumors are seen in those aged less than 18 years.[10] Factors associated with GBM risk are prior radiation, decreased susceptibility to allergy, immune factors and immune genes, and some nucleotide polymorphisms, detected by genome-wide association.[11,12] The lower risk of GBM in people with asthma and other allergic conditions is consistent with findings that have been confirmed by objective evidence from asthma and other allergy-related germline polymorphism in patients with GBM and in controls. Genotypes that increase asthma risk are associated with decreased GBM risk.[12] GBM is an aggressive neoplasm with a median survival of only 3 months in untreated patients.[13] Surgery remains an important component in the management of GBM. Surgery enables a histological confirmation of the clinical diagnosis and also has decompressive and cytoreductive effects, with the advantage of increased survival with complete resection.[14] Tumor fluorescence derived from five aminolevulinic acid enabled a more complete resection of contrast-enhancing tumors, leading to improved progression-free-survival in patients with GBM.[14] The main contraindications to resective surgery are poor performance status (Karnofsky of less than 70), advanced age, and eloquent location.[15] The combination of radiotherapy and TMZ chemotherapy is the most effective adjuvant therapy shown to prolong survival following primary resection.
Radiotherapy followed by TMZ results in significantly prolonged survival compared with radiotherapy alone. Treatment of GBM remains challenging. The current experience in GBM treatment shows that several targets should be approached. Therefore, rational combinations between established treatments and new approaches aiming, for example, at inhibition of angiogenesis, induction of apoptosis, or inhibition of several signal transduction pathways might offer the best opportunity to improve prognosis.
MATERIAL AND METHODS
This was a retrospective study performed in the Department of Radiation Oncology, Sher-I-Kashmir Institute of Medical Sciences, Soura, Srinagar, India. All necessary clinical and epidemiological details of the 80 cases diagnosed with glioblastoma multiforme from January 2016 to December 2020 were retrieved. Clinical and epidemiological features, treatment, overall survival and follow-up data were analyzed.
Statistical analysis
The data were first keyed into a Microsoft Excel spreadsheet and cleaned for any inaccuracies. Statistical analysis was done using IBM SPSS Statistics for Windows from IBM Corp. (released 2020, Version 27.0. Armonk, NY, USA). Categorical variables were shown in the form of frequencies and percentages.
Ethics
The procedure in conducting the study was as per the institutional ethics committee guidelines and as per the Helsinki Declaration of 1964, revised in 2013. Informed consent was waived, as this was a retrospective audit of the health records.
RESULTS
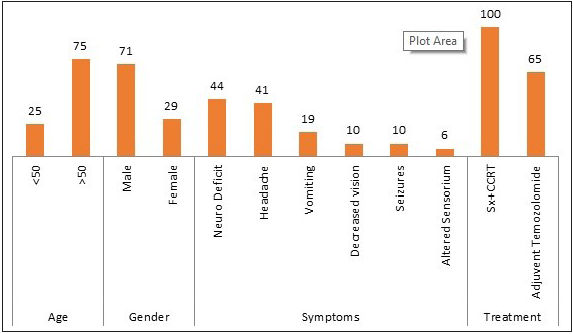
In the current study, a total of 80 patients were analyzed. The mean age of diagnosis was 51 years, with ages ranging from 25 to 70 years. Most patients were male 57 (71%), and the incidence rate was higher in male patients than female patients with a male:female ratio of 2:1. Neurodeficiency and headache were the most common presenting symptoms. Other symptoms include vomiting, decreased vision, seizures, and altered sensorium. The most common site of tumor was the fronto-parietal region followed by the occipito-temporal region [Table 1].
Abbreviations: GBM, Glioblastoma Multiforme; Sx, Surgery; CCRT, Concurrent Chemoradiation.
Variables
Category
N = 80
%
Age
<50
20
25
>50
60
75
Gender
Male
57
71
Female
23
29
Symptoms
Neuro deficit
35
44
Headache
33
41
Vomiting
15
19
Decreased vision
8
10
Seizures
8
10
Altered sensorium
5
6
Treatment
Sx+CCRT
80
100
Adjuvent temozolomide
52
65
All patients underwent surgical debulking followed 6–8 weeks by concurrent chemoradiation to a dose of 60 Gy in 30 fractions @ 2 Gy per fraction over a period of 6 weeks, with 5 fractions per week concurrent with temozolomide (75 mg/m 2 ). Intensity modulated radiotherapy (IMRT) was the most common technique used, particularly in younger patients, followed by three-dimensional conformal radiotherapy (3DCRT) and conventional techniques. There was no survival benefit using different radiotherapy techniques, but the toxicity profile was lower with the IMRT technique. Headache, hair loss, vomiting, somnolence, fatigue, and cognitive decline were the various side effects seen in these patients while undergoing radiation.
Most of the patients, almost 80–90% had residual diseases even after completing chemoradiation and were subjected to adjuvant chemotherapy, i.e., temozolomide 250 mg for 6 cycles. 25 patients (31%) died within 8 months of diagnosis, 45 patients (56%) within 9–15 months, and 10 patients (13%) survived for more than 16 months [Figure 1].
- Profile of GBM.
DISCUSSION
Glioblastoma multiforme is the most aggressive primary central nervous system (CNS) neoplasm. These neoplasms usually occur in the sixth and seventh decades of life.[16,17] In the present study, the median age of our patients was 53 years, which was consistent with the results of the literature review, in which the average median age of 7,726 patients in the reported series was 62 years.[18 – 23] In almost all reported series in the literature review, men represent a higher proportion of GBM sufferers than women, with a mean male/female ratio of 1.4 (range from 1.0 to 1.9) in eight studies including 4,933 patients.[24 – 27] In the present study, this ratio was 2:1 which was consistent with the reported series. Glioblastoma multiforme are diffusely infiltrative tumors; consequently, surgical curative resection is rarely possible for this neoplasm. Optimal safe resection is an essential goal in the initial management of patients with GBM, and the extent of surgical resection must be balanced against the risk of neurologic dysfunction. A variety of preoperative neuroimaging and intraoperative mapping and neuromonitoring techniques have been incorporated into patient management to achieve these goals.
Postoperative adjuvant radiotherapy is a principal element in the treatment of patients with GBM.[28] External beam radiotherapy is usually recommended to start within 2–4 weeks following surgical resection or biopsy. A total dose of 60 Gy is often delivered using involved portals and conventional fractionation (daily fractions of 2 Gy, five fractions per day).[28 – 30] Adjuvant chemotherapy plays an important role in the management of patients with GBM.[23,28] Before 1999, nitrosourea-based combinations were the most commonly used chemotherapeutic agents in GBM, among which carmustine and lomustine were the most active agents. However, by adding these agents to combined surgery and postoperative radiotherapy, no significant improvements in terms of response rates and overall survival were observed.[28,31] Since 1999 and by introducing temozolomide a modest improvement in median survival has been seen. At present, concurrent chemoradiation followed by sequential adjuvant temozolomide is recommended for patients with GBM.[28,32,33]
Glioblastoma multiforme is a highly aggressive tumor, median survival is usually less than 12 months, and long-term survival is exceptional.[27,34] In almost all reported series in the literature, we found young age, good performance status, and safe optimal resection to be the well-known good prognostic factors in patients with GBM. In the present study, we found radiation dose, extent of surgical resection, and adjuvant chemotherapy to be independent prognostic factors for overall survival. Mutations of tumor suppressor genes, particularly p53 and amplifications of oncogenes, especially EGFR gene amplification, play an important role in the pathogenesis and progression of GBM. In this series, there was no data regarding molecular markers, and these markers are not routinely checked in our patients with GBM.
CONCLUSION
According to the findings of the present study and review of the literature, GBM is a highly violent tumor; tends to have early relapse and short-term survival. Multimodality therapy, including safe optimal surgical resection combined with adjuvant radiotherapy or concurrent chemoradiation and sequential chemotherapy, is recommended for all patients with this fatal neoplasm. Despite modest improvement in the overall survival of patients with GBM in the recent decade, the outcome remains poor. Therefore, the need for more effective novel treatments for this neoplasm is urgently needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Sheri Kashmir Institute of Medical Sciences, Srinagar, UT J&K, India.
Conflicts of interest
None declared.
REFERENCES
- The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803-20.
- [CrossRef] [PubMed] [Google Scholar]
- CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15 Suppl:2ii-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Improved survival time trends of glioblastoma using the SEER 17 population-based registries. J Neuro Oncol. 2012;107:207-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Radiotherapy plus concomitant and adjuvant Temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
- [CrossRef] [PubMed] [Google Scholar]
- A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104:2798-806.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equine. J Neurosurg Spine. 2010;13:67-77.
- [CrossRef] [PubMed] [Google Scholar]
- Adult cerebellar glioblastoma: Understanding survival and prognostic factors using a population-based database from 1973–2009. World Neurosurg. 2013;80:e181-3.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome and prognostic factors in adult cerebellar glioblastoma. J Clin Neurosci. 2013;20:1117-21.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of survival between cerebellar and supratentorial glioblastoma patients: Surveillance, epidemiology, and end results (SEER) analysis. Neurosurgery. 2013;73:240-6.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive factors determining the overall outcome of primary spinal glioblastoma multiforme: An integrative survival analysis. World Neurosurg. 2016;86:341-8.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular epidemiology of gliomas in adults. Neurosurgical Focus. 2005;19:1-11.
- [CrossRef] [PubMed Central] [Google Scholar]
- Epidemiology and molecular pathology of Glioblastoma multiforme. Nat Clin Pract Neurol. 2006;2:494-503.
- [CrossRef] [PubMed] [Google Scholar]
- Temozolamide versus standard 6-week radiotherapy versus hypofractionates radiotherapy in patients older than 60 years with glioblastoma.The Nordic randomized phase 3 trial. Lancet Oncol. 2012;13:916-26.
- [CrossRef] [PubMed] [Google Scholar]
- Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62:564-76.
- [CrossRef] [PubMed] [Google Scholar]
- Glioblastoma multiforme: State of art and future therapeutics. Surg Neurol Int. 2014;5:64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gliomas in adults. Dtsch Arztebl Int. 2010;107:799-807.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic factors other than the performance status and age for glioblastoma multiforme: a single-institution experience. J BUON. 2009;14:211-8.
- [PubMed] [Google Scholar]
- Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114:587-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112:997-1004.
- [CrossRef] [PubMed] [Google Scholar]
- Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol. 2011;103:611-8.
- [CrossRef] [PubMed] [Google Scholar]
- Survival improvement in patients with glioblastoma multiforme during the last 20 years in a single tertiary-care center. Wien Klin Wochenschr. 2003;115:389-97.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol. 2003;21:1485-91.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin Med J (Engl). 2009;122:1245-9.
- [PubMed] [Google Scholar]
- Prognostic impact of hemoglobin level prior to radiotherapy on survival in patients with glioblastoma. Strahlenther Onkol. 2003;179:615-9.
- [CrossRef] [PubMed] [Google Scholar]
- A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061-83.
- [CrossRef] [PubMed] [Google Scholar]
- The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro Oncol. 2010;12:190-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Irradiation fields and doses in glioblastoma multiforme: are current standards adequate? Tumori. 2001;87:85-90.
- [CrossRef] [PubMed] [Google Scholar]
- PCV chemotherapy for recurrent glioblastoma multiforme. Neurology. 2001;56:118-20.
- [CrossRef] [PubMed] [Google Scholar]
- Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13:537-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Radiochemotherapy with temozolomide for patients with glioblastoma. Prognostic factors and long-term outcome of unselected patients from a single institution. Strahlenther Onkol. 2011;187:722-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival with glioblastoma multiforme. Brain. 2007;130(Pt 10):2596-606.
- [CrossRef] [PubMed] [Google Scholar]







