Translate this page into:
Cutaneous Metastasis: A Prelude to a Sinister Outcome
Address for correspondence Vikas Pathania, MD, Department of Dermatology, Armed Forces Medical College, Pune 411040, Maharashtra, India. vikascongo@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cutaneous metastasis is an uncommon presentation of primary internal malignancy presenting for dermatology cross-referrals with an incidence ranging from 0.7 to 10%. It may be the first presentation of an underlying primary malignancy. The primary may remain unknown in up to 3 to 5% cases. Detailed evaluation of clinical presentation with emphasis on cytomorphological features, immunohistochemistry, and imaging aids in identification of an unknown primary. Incidence of leukemia cutis has been reported to be as high as 22% and is associated with poor prognosis. In majority of the patients, presentation of cutaneous metastasis signifies advanced disease with ominous outcome. It correlates with either treatment failure or reoccurrence of previously treated malignancy. Though cutaneous metastatic lesions are amenable to early visual detection, this entity may be easily mistaken for common dermatological conditions and hence goes unrecognized. Lesions of cutaneous metastasis may bear therapeutic and prognostic implications. Therefore, a very high index of suspicion is warranted to accurately and timely diagnose such manifestations of hidden malignancies. We report three such cases that highlight the protean manifestations of internal malignancies.
Keywords
adenocarcinoma
cutaneous metastasis
carcinoma of unknown primary
malignancy
Introduction
Cutaneous metastasis from underlying malignancy is a rare presentation with incidence ranging from 0.7 to 10%.1,2 Majority of these occur in breast, lung, and colon malignancies. Most cutaneous metastasis are seen with a known primary; however, these may be the first manifestation of an underlying simmering neoplasia in 0.8 to 22% of the cases.3,4 In 3 to 5% of cases, the origin of primary may remain unknown.5
These being a hallmark of widely spread primary malignancy portends a poor prognosis with half of the patients dying within 6 months of developing cutaneous metastasis.6
Case 1
A 64-year-old female being managed as a case of carcinoma of unknown primary site (CUPS) presented with rash over both retroauricular regions and neck of 6 weeks duration. One year prior to onset of this rash, she presented with right-sided neck swelling. A biopsy of right level II cervical lymph node (LN) revealed evidence of metastatic deposits of poorly differentiated grade 3 adenocarcinoma. An extensive search for occult primary cancer was done considering the European Society for Medical Oncology group recommendations.5 Tumor markers for breast (estrogen receptor–progesterone receptor), carcinoembryonic antigen were positive and positron emission tomography-computed tomography (PET-CT) showed increased fluorodeoxyglucose (FDG) uptake of right cervical LN levels II to V. She was being managed with concurrent chemotherapy (paclitaxel and carboplatin) and radiotherapy for 06 cycles when she developed red raised rash over both cheeks, retroauricular regions, and neck associated with itching and burning sensation with gradual hardening of overlying skin.
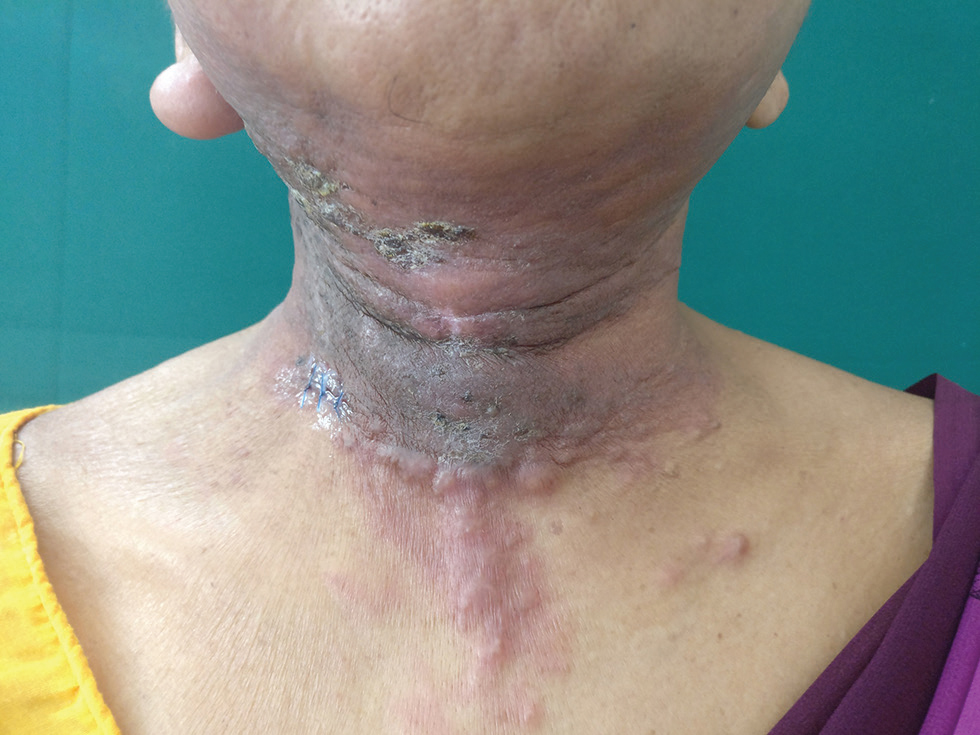
Examination revealed diffuse erythematous indurated plaque with adherent crusts at places involving both retroauricular regions, both cheeks, anterior aspect of neck, submental and submandibular region, and upper chest. Multiple nontender indurated firm nodules were overlying this plaque. There was swelling of right ear lobe (Fig. 1). Left inferior axillary LN was palpable that was 1 cm in size, nontender, firm, and freely mobile. Skin biopsy done from neck revealed epidermis comprised of keratinized stratified squamous epithelium. Dermis showed numerous nests of atypical cells in a fibrocollagenous stroma (Fig. 2A). Cells were large with high nuclear cytoplasmic ratio, pleomorphic nuclei with prominent nucleoli (Fig. 2B). Immunohistochemistry (IHC) revealed atypical cells positive for CK7, CK20, GCDFP, and CDX2 (Fig. 2C–F, respectively). A repeat PET-CT revealed two new FDG avid axillary LN without any localization to breast, lung, colorectal, or ovarian tissue. The patient was referred to oncologist for further management.
-
Fig. 1 Case 1: Diffuse erythematous indurated plaque with adherent crust at places seen over submental and submandibular region, anterior aspect of neck, and “V” area of chest. Multiple nontender indurated firm nodules seen overlying this plaque. Swollen right ear lobe is also seen.
![Fig. 2 (A) Dermis shows presence of numerous nests of atypical cells in a fibrocollagenous stroma (yellow star; hematoxylin & eosin [H&E] 100×). (B) Cells are large with high nuclear cytoplasmic ratio, pleomorphic nuclei with prominent nucleoli. No necrosis/mitosis is noted (H&E 400×). (C) Immunohistochemistry (IHC) CK7 (100×). (D) IHC CK20 (100×). (E) IHC GCDFP (100×). (F) IHC CDX2 (100×).](/content/155/2020/06/01/img/10-1055-s-0040-1702909_00051_02.png)
-
Fig. 2 (A) Dermis shows presence of numerous nests of atypical cells in a fibrocollagenous stroma (yellow star; hematoxylin & eosin [H&E] 100×). (B) Cells are large with high nuclear cytoplasmic ratio, pleomorphic nuclei with prominent nucleoli. No necrosis/mitosis is noted (H&E 400×). (C) Immunohistochemistry (IHC) CK7 (100×). (D) IHC CK20 (100×). (E) IHC GCDFP (100×). (F) IHC CDX2 (100×).
Case 2
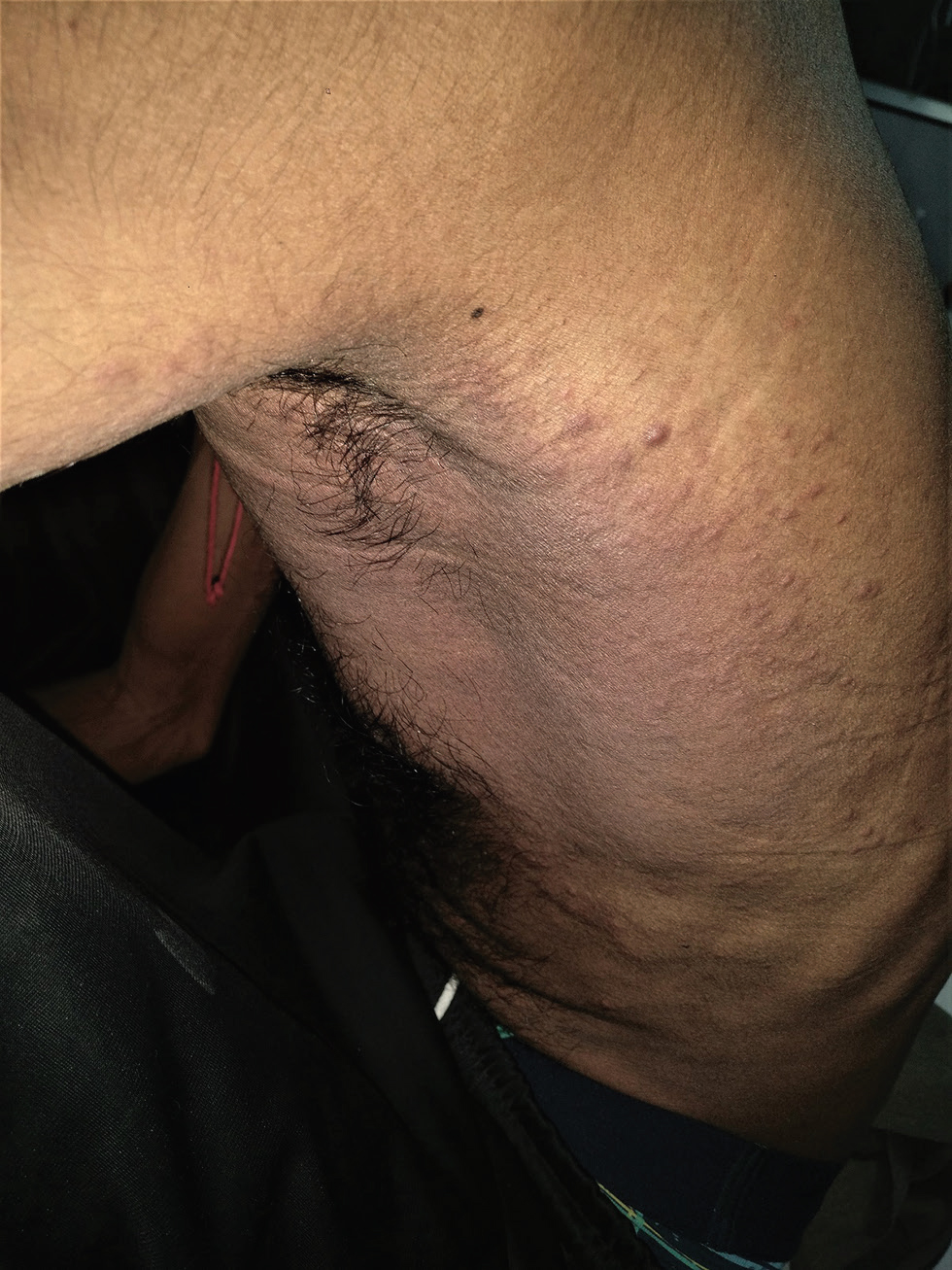
A 33-year-old male, with a diagnosed case of metastatic carcinoma of the gastroesophageal junction T3N3M1, having received one cycle of chemotherapy (oxaliplatin, epirubicin, capecitabine), presented with diffuse redness of left infra-axillary region of 7 days duration. On examination, there was diffuse erythema and edema over left infra-axillary region seen extending posteriorly with multiple overlying erythematous papules and plaques (Fig. 3). Skin biopsy was suggestive of cutaneous metastasis with predominantly malignant adenocarcinomatous morphology (Fig. 4AC). Local radiotherapy was added subsequently but the patient died 2 months later.
-
Fig. 3 Case 2: Diffuse erythema and edema over left infra-axillary region extending posteriorly with multiple overlying erythematous papules and plaques are seen.
![Fig. 4 (A) Skin biopsy reveals focal collections of intermediate-to-large sized cells in the dermis particularly around perivascular areas (yellow star; hematoxylin & eosin [H&E], 100x). (B) The malignant cells lying singly in small nests, attempting to form acini (yellow circle) (H&E, 200x). (C) Malignant cells are anaplastic with large irregular hyperchromatic nuclei (red arrow) (H&E, 400x).](/content/155/2020/06/01/img/10-1055-s-0040-1702909_00051_04.png)
-
Fig. 4 (A) Skin biopsy reveals focal collections of intermediate-to-large sized cells in the dermis particularly around perivascular areas (yellow star; hematoxylin & eosin [H&E], 100x). (B) The malignant cells lying singly in small nests, attempting to form acini (yellow circle) (H&E, 200x). (C) Malignant cells are anaplastic with large irregular hyperchromatic nuclei (red arrow) (H&E, 400x).
Case 3
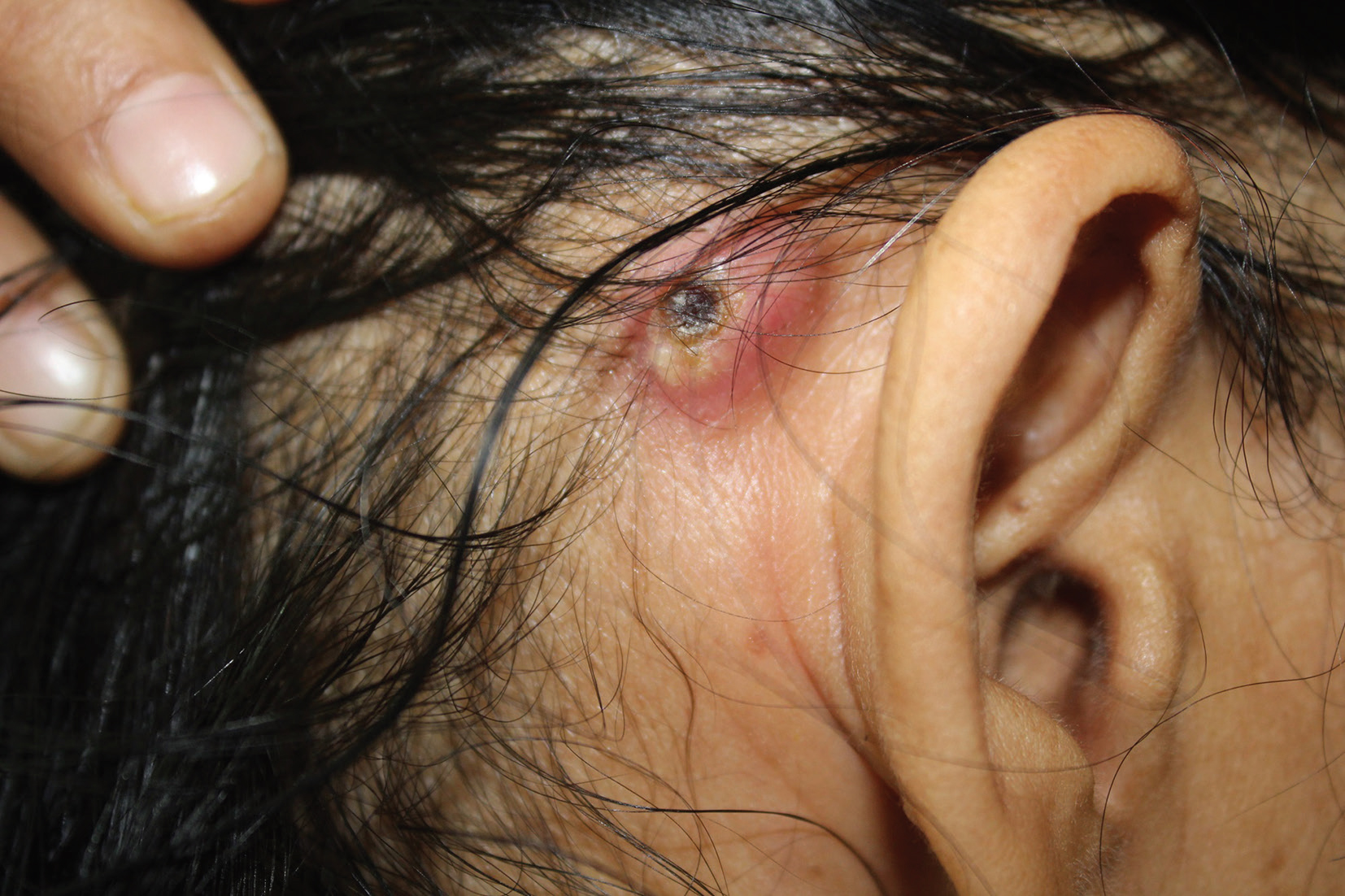
A known case of acute myeloid leukemia (AML) with monosomy 7 having received three cycles of chemotherapy (idarubicin, cytarabine, azacytidine) developed painful retroauricular swellings of 7 days duration. Dermatological examination revealed discrete firm and tender nodules with central crust involving both retroauricular regions symmetrically with extrusion of purulent discharge (Fig. 5). Skin biopsy done from a nodule revealed dermal vessels with extravasated atypical cells that were CD34 and CD117 positive on IHC (Fig. 6AC). These were consistent with cutaneous myeloid precursors. Patient was administered fourth cycle of chemotherapy. However, he succumbed to his illness a month later.
-
Fig. 5 Case 3: Discrete solitary nodule with central crust seen over right retroauricular region.
![Fig. 6 (A) Dermis shows superficial dermal vessels with few extravasated large atypical cells (black arrow; hematoxylin & eosin [H&E] 400x). (B) Immunohistochemistry (IHC) CD34 positive (100x). (C) IHC CD117 positive (100x).](/content/155/2020/06/01/img/10-1055-s-0040-1702909_00051_06.png)
-
Fig. 6 (A) Dermis shows superficial dermal vessels with few extravasated large atypical cells (black arrow; hematoxylin & eosin [H&E] 400x). (B) Immunohistochemistry (IHC) CD34 positive (100x). (C) IHC CD117 positive (100x).
Discussion
Cutaneous metastasis from an underlying visceral malignancy is an infrequent occurrence with incidence ranging from 0.7 to 10%.1,2 Krathen et al have reported an incidence of 5.3% among 1,080 patients of their total pool of 20,380 cancer patients.7 Any cancer can develop cutaneous metastasis. Most presentations are associated with breast, lung, colorectal cancer, and melanoma with overall breast cancer being the commonest.2,7,8 In males, lung carcinoma is the commonest primary reported with skin metastasis.9
Cutaneous metastasis occurs as a result of detached tumor cells from occult primary by direct invasion, locoregional invasion through body cavities or lymphatics, and distant metastasis by hematogenous or lymphatic route. Exact molecular mechanism of spread is complex with role of certain chemokines, their receptors, and interactions between tumor cells and epidermal–dermal factors.9 Immunological milieu of normal skin may partly determine the relative location of cutaneous metastasis.8 Commonest locations are head and neck region and scalp followed by chest and abdomen.8,9 Involvement of head and neck region frequently could be attributed to relatively high Treg density and increased CD4:CD8 ratio that is thought to be most amenable to tumor growth.8 Chest wall is favored site for lung and breast carcinoma, abdomen for gastrointestinal tumors, and back for renal cell cancer (RCC). This signifies the fact that site of skin involvement can possibly hint at underlying organ involvement.9 In contrast, metastatic lesions in leukemia cutis (LC) have no particular anatomic preference.
Various presentations of cutaneous metastasis include a gamut ranging from solitary to multiple skin colored dermal or subcutaneous nodules to inflammatory lesions including erythematous infiltrating plaques, alopecia neoplastica, telangiectatic violaceous papules and vesicles, zosteriform lesions, pulsatile red/purple-colored nodules in RCC, and blanchable bluish nodules in neuroblastoma.9 It may be the first presentation of an underlying primary malignancy in 0.8 to 22%.3,4
CUPS is a fascinating phenomenon seen in 3 to 5% of all cases where origin of primary may remain unknown.5 It is defined as histologically confirmed metastasis in the absence of an identifiable primary tumor, despite exploration with standardized diagnostic approach as was done in our index case.5 Dormancy of occult primary, early metastasis, and treatment resistance is hallmark of CUPS.
Incidence of LC has been reported to be as high as 22%.10 Its presentation suggests other sites of extramedullary involvement pointing to advanced stage of illness with poorer prognosis. Morphologically, LC presents as nonspecific lesions from erythematous papules and/or nodules, infiltrated plaques, palpable purpura, ulcers, ecchymoses, and/or vesicles.
Clinically, cutaneous metastasis may mimic primary adnexal tumor. Distinction between these entities and correct diagnosis is by detailed histo-cytological evaluation including assays for markers and IHC. Cytological diagnosis using immunostains helps to suggest possible origin of primary tumor especially in CUPS and additionally confirms previous known malignancy. Morphologically, these are classified as nodular, infiltrative, diffuse, or intravascular and top heavy or bottom heavy. Top-heavy and bottom-heavy patterns refer to cellular infiltrates with a large base in the superficial or deeper part of dermis, respectively.11 Metastasis is classified as adenocarcinoma, squamous cell carcinoma, or undifferentiated (anaplastic) with adenocarcinoma being the commonest reported entity. Two of our patients had adenocarcinoma.
Working algorithms involving desired set of immunostains are recommended for cytological analysis. Fine-needle aspiration cytology of metastatic cutaneous lesion has been suggested as the first-line rapid and safe technique for confirmation.9 Ultimately, combination of histo-cytology, detailed clinical examination, and imaging aids in clinching the diagnosis of primary malignancy, especially in CUPS.
Treatment generally follows the regimen appropriate for primary metastatic malignancy. However, systemic therapy has limited efficacy.12 Skin-directed therapies have been recommended to manage cutaneous metastasis that elicit higher local response and low recurrence rates than systemic therapy. These include electrochemotherapy, cryotherapy, photodynamic therapy, radiation, intralesional, and topical therapy. Electrochemotherapy appears to be the most efficacious that involves application of pulsed electricity to a tumor-specific area thereby increasing cell permeability.12 Treatment modalities used in LC include chemotherapy with daunorubicin, cytarabine, retinoic acid, rituximab, etoposide, methotrexate, and neomycin. However, previous reports have shown limited response to therapy. Escape of cutaneous tumor cells from effect of chemotherapy has been proposed as explanation for poor prognosis in AML patients with LC as most chemotherapeutics mainly target bone marrow tumor cells. Therefore, total skin electron beam radiation therapy targeting cutaneous tumor cells specifically may be used concurrently in LC.10 Complete remission with the use of sorafenib has been reported in a case of LC.13 Immunotherapy with programmed cell death ligand 1 inhibitor and talimogene lahirparepvec has demonstrated efficacy in the treatment of melanoma-associated cutaneous metastasis.14,15
Outcome in cases with cutaneous metastasis is generally poor with mortality in half of the patients within 6 months of initial diagnosis. Schoenlaub et al have reported median survival after cutaneous metastasis as 6.5 months with worst prognosis for lung carcinoma with median survival of 2.9 months.6 In LC in AML, most patients succumbed to illness within a year of diagnosis.
Conclusion
Cutaneous metastasis in majority of patients signifies advanced disease with grave outcome. It correlates with either treatment failure or reoccurrence of previously treated malignancy. Any unexplained persistent erythema, induration, and skin lesions of unknown cause must be investigated and biopsied to rule out cutaneous metastasis as it may be a pointer to an undiagnosed primary malignancy or may signify ominous sign of systemic metastasis. Cutaneous metastatic lesions may, therefore, have therapeutic and prognostic implications. A high index of suspicion is warranted to timely diagnose such manifestations of hidden malignancies with a combination of detailed clinical, histo-cytological, and imaging evaluation.
Conflict of Interest
None declared.
References
- Malignant tumors and their metastases: a summary of the necropsies on eight hundred sixty-five cases performed at the Bellevue Hospital of New York. Ann Surg. 1930;92(03):434-443.
- [Google Scholar]
- Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236. (2 Pt 1)
- [Google Scholar]
- Skin metastases as the first manifestation of lung cancer: A case report. Int J Res Heal Sci. 2014;2:363-365.
- [Google Scholar]
- Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135(01):1-14.
- [Google Scholar]
- Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(05):v133-v138.
- [Google Scholar]
- [Survival after cutaneous metastasis: a study of 200 cases] Ann Dermatol Venereol. 2001;128(12):1310-1315.
- [Google Scholar]
- The distribution of cutaneous metastases correlates with local immunologic milieu. J Am Acad Dermatol. 2016;74(03):470-476.
- [Google Scholar]
- Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61(01):47-54.
- [Google Scholar]
- Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51(04):383-388.
- [Google Scholar]
- Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32(03):222-239.
- [Google Scholar]
- Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32(28):3144-3155.
- [Google Scholar]
- Complete resolution of leukemia cutis with sorafenib in an acute myeloid leukemia patient with FLT3-ITD mutation. Am J Hematol. 2009;84(10):701-702.
- [Google Scholar]
- Inflammatory melanoma in transit metastases with complete response to talimogene laherparepvec. JAAD Case Rep. 2017;3(04):280-283.
- [Google Scholar]
- Definite regression of cutaneous melanoma metastases upon addition of topical contact sensitizer diphencyprone to immune checkpoint inhibitor treatment. Exp Dermatol. 2016;25(07):553-554.
- [Google Scholar]








