Translate this page into:
A Radiological Curiosity of a Rare Diagnosis: Lhermitte-Duclos Disease
Address for correspondence Wilson Bizimana, MD, Department of Radiology, UHC Ibn SINA, Rabat 8083, Morocco. wilson.bizimana@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lhermitte–Duclos disease (LDD) is a rare cerebellar lesion, described in 1920 by two French physicians: Lhermitte and Duclos. The clinical presentation is usually made of neurological symptoms. This lesion is characterized by a hamartomatous lesion in the posterior fossa. Mainly diagnosed by MRI, when it comes to preoperative, the T2-weightened MRI demonstrates the classical “tiger-striped” pattern. The definitive diagnosis, nonetheless, is histopathological. The treatment for LDD consists of surgical decompression or excision. We present here a rare case of a woman who developed neurological symptoms that led to LDD diagnosis to describe protocol MRI imaging, the main findings and their pathophysiological meanings.
Keywords
Lhermitte Duclos disease
tiger striping sign
cerebellum
Introduction
Lhermitte Duclos disease is a dysplastic gangliocytoma. The incidence is approximately 5 per million per year.1 It is an extremely rare benign tumor of specific cerebellar localization, with poorly understood etiopathogeny.2 It was first described in 1920 by two French people, Lhermitte and Duclos.3 There is a recognition of a higher female preponderance.4 Magnetic resonance imaging can identify its characteristic image due to a distortion of the laminar architecture of the cerebellum and impregnating a tiger striped and flaky appearance to the lesion area of the cerebellum. The treatment is a simple monitoring if the patient is asymptomatic or decompression excision surgery when the patient is hyper symptomatic.
Patient and Observation
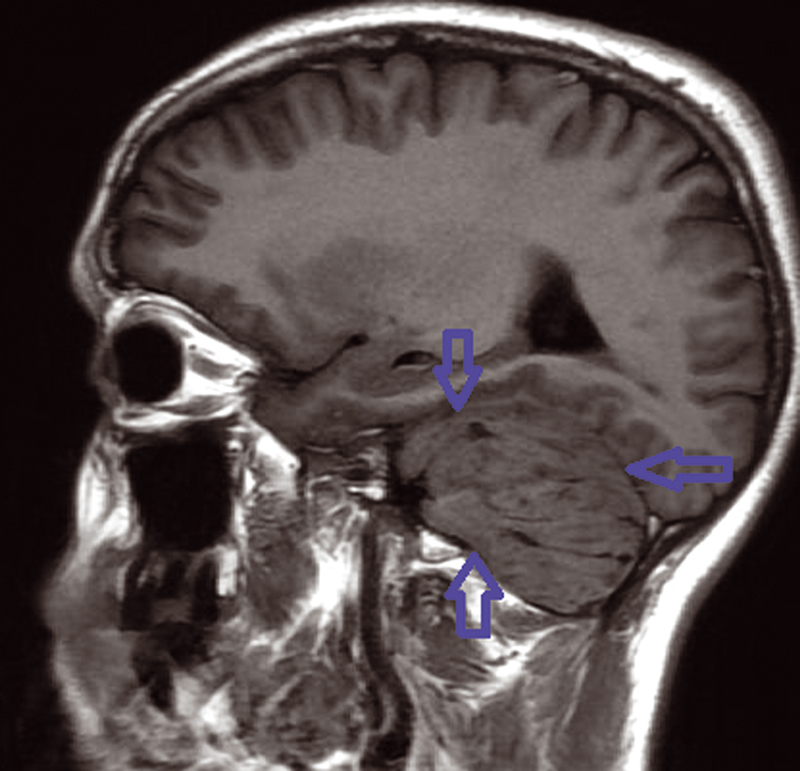
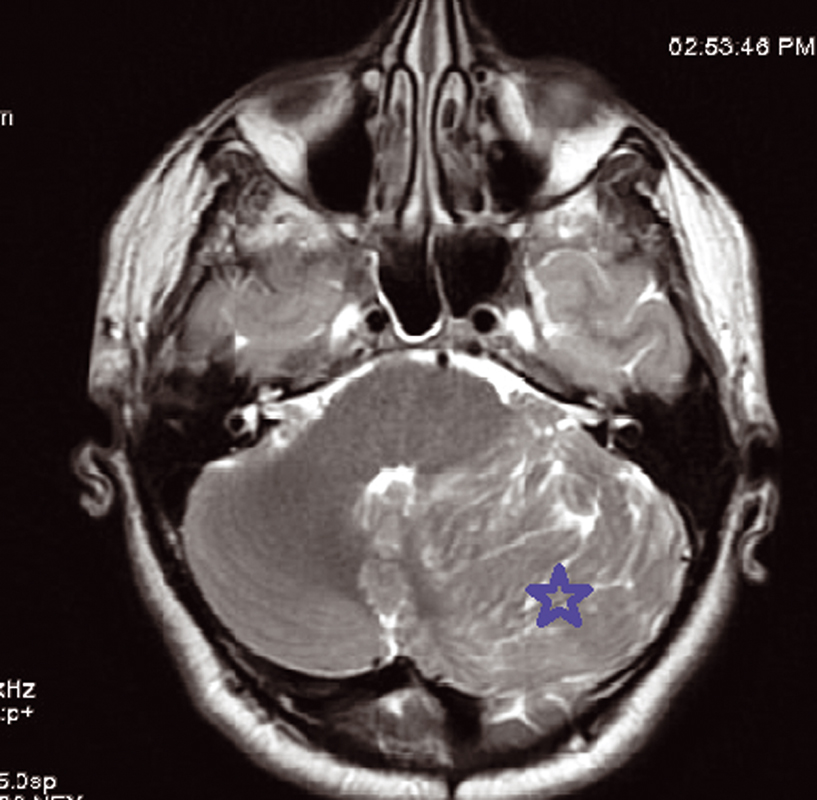
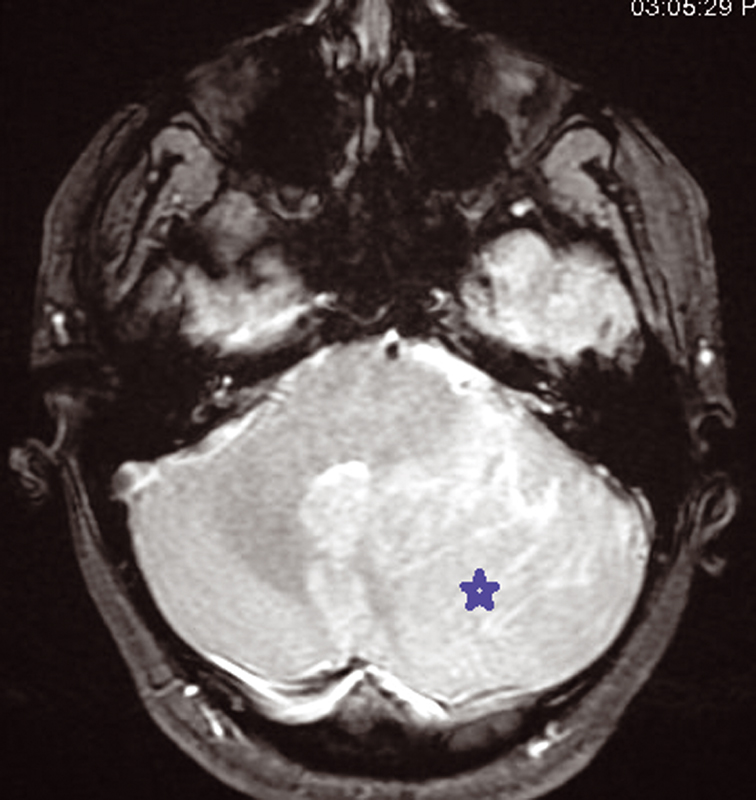
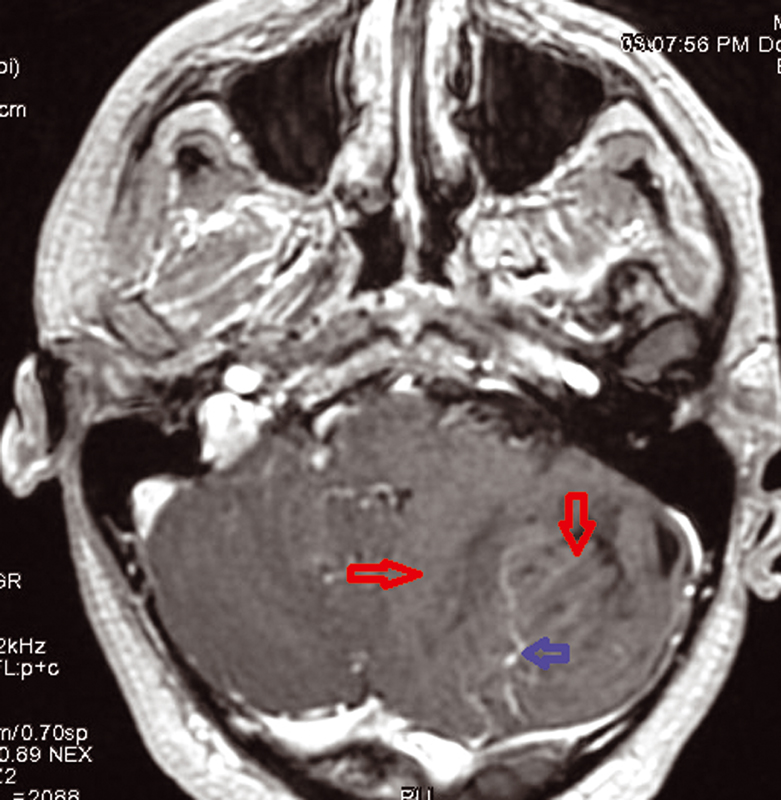
We present a 30-year-old women patient who is admitted for headache and sometimes vomiting .Faced with this meningeal syndrome, a brain CT scan was done in 2019 noting a heterogeneous hypodensity of the left cerebellar hemisphere without any signs of subarachnoid hemorrhage. A Brain MRI done in August 2020 showed the presence on the left cerebellar hemisphere, an alternation of roughly parallel linear bands in net hypo-signal T1, high signal T2, T2 flair and diffusion with non-enhancement after injection of Gadovist .The lesion produced a mass effect on the vermis, the fourth ventricle, and on the punt and it measured 59 × 60 × 68 mm. The MRI did not note any abnormality in the supratentorial stage or hydrocephaly or other malformation of the posterior cerebral fossa. The conduct performed consisted of a craniotomy for a biopsy performed in the Neurosurgery department and finally the anatomopathologist concluded a dysplastic gangliocytoma of the cerebellum. One year later, i.e., in July 2021, the same symptomatology associated with visual disturbances reappeared. Imaging control was requested from the same central radiology department and the following sequences were performed, T1 sequence, T2 flair sequence, sequence of diffusion, echo of gradient (T2*), T1FS + gadolinium. MRI found at the level of the left cerebellar hemisphere, a mass of striated, lamellar appearance formed by the alternation of linear bands roughly parallel in net hypo-intensity T1 (Fig. 1), hyperintense T2, and flair (Fig. 2)with weak restriction of the diffusion in some zones (Fig. 3) and presenting a weak venous enhancement draining into the left lateral sinus (Fig. 4). This time, it was measuring 71 × 77 × 50 mm (AP × T × H). Laterally, this process exerted a mass effect on the vermis, the fourth ventricle and the punt with no hydrocephalus upstream (Figs. 2 and 4). The comparison with the anterior exam concluded that dysplastic gangliocytoma of the left cerebellum was relatively stable. In view of clinical imaging, the patient was referred to Neurosurgery Department and scheduled for RCP to discuss symptomatic treatment with monitoring or outright surgery excision of the mass.
-
Fig. 1 Left mass cerebellum in T1-weighted sequence MRI.
-
Fig. 2 Hyperintensity intralesional on T2 sequence MRI showing the typical striated or tiger-striped folia pattern.
-
Fig. 3 Hyperintensity intralesional on diffusion sequence MRI.
-
Fig. 4 Absence of enhancement intralesional with venous abnormalities on T1 + Gado sequence MRI.
Discussion
Lhermitte-Duclos disease, or dysplastic cerebellar gangliocytoma, is a rare hamartomatous lesion due to abnormal development and unilateral hemispheric expansion of the cerebellum.5 Dysplastic cerebellar gangliocytoma is seen in adult and children, most frequently in young adults in the thirties.6 Clinically, patients may be asymptomatic, or they may present with symptoms and signs of increased intracranial pressure. Cranial nerve palsies, cerebellar symptoms, gait ataxia, and sudden neurological deterioration as a result of occlusive hydrocephalus are frequent findings.6,7
Imaging Findings
On a CT examination, dysplastic cerebellar gangliocytoma presents as a low-density cerebellar mass, which may contain calcification and does not show enhancement.8 MRI is the best imaging performed to diagnose Lhermitte–Duclos disease (LDD). The MRI protocol is standard: axial, sagittal, and coronal planes are required. The most common sequences are T1, T2, Flair, DWI (b1000), the Injection of Gadolinium and spectroscopy at 135m/s, MRI checks for architectural disorganization and cortical hypertrophy, and loss of parallelism of the cerebellar lamellae or folia.3
The characteristic of tiger striped and flaky appearance is found in T2 FSE and T2 Flair in the form of leafy and tabby mass syndrome of the cerebellar hemisphere. The involvement is often unilateral, very rarely bilateral, only one case reported in literature.9 In T1-weighted image, there is distortion of foliar architecture of cerebellum in isointense or hypointense compared with gray substance (Figs. 1 and 5). T2 Flair, T2-weighted axial, and coronal imaging revealed a well-circumscribed hyperintense mass with lamellar areas of isointensity which occurs as a result of enlargement of cerebellar folia and compressed sulci (Fig. 2). After injection of gadolinium (T1FS + Gado), two figures can be observed: either a hypervascularization compared with the healthy contralateral parenchyma, a situation which is very rare, in connection with venous abnormalities of development and not an angiogenesis or an absence of contrast enhancement which proves that post-gadolinium-enhanced axial T1-weighted image demonstrates no significant enhancement within the mass testifying to the integrity of the blood–brain barrier (Fig. 4).10,11 In diffusion it is identified by a global hyperintensity of the lesional zone with an alternation of the zones in restriction of diffusion and absence of restriction of diffusion confirmed by the ADC cartography(Fig. 3). Diffusion properties in LDD can be variable depending on the contributions from the inner layer and the thick outer molecular layer with large dysplastic neurons.12 This testifies to hypercellularity and the high intralesional axonal concentration.13
-
Fig. 5 Right cerebellum hemisphere normal in T1-weighted sequence MRI.
Spectroscopy sequence shows a non-specific drop in NAA with inverted lactate peak and reduction in choline. An absence of an increase in choline leading to a drop in the NAA/Cr and Cho/Cr ratio, reflects the absence of aggressiveness of a tumor in the majority of cases.12,14 Mass syndrome has a mass effect most often on the fourth ventricle and sometimes on the protuberance. This increase in the cerebellar mass ratio is related to an abundance of dilated intra- and perilesional veins, in the altered cell layers and along the leptomeninges. The abnormality is hemispheric and unilateral most often but only one case of bilaterality has been reported.9,15 In sum, the typical striated or tiger-striped folia pattern is a characteristic finding of LDD also named dysplastic gangliocytoma.16
The radiologist must be careful with the medulloblastoma which can mimic this diagnosis by rarely showing a resemblance of these morphological characteristics except that in the latter we observe a peak of choline on spectroscopy.13,14 From the above, histological proof is inescapable. Histological hallmarks of the disease are thickening and hypermyelination of the outer molecular layer, loss of Purkinje cells and white matter, dysplastic ganglion cells with rounded nuclei, and abundant mitochondria invading the inner granular layer.2,5
Recurrences are possible in case of incomplete excision and a syndromic association with Cowden disease is frequently found involving LDD, multiple skin lesions, and cancers (breast, thyroid, digestive, and genitourinary). In this Cowden disease, LDD represents the neurological expression of this rare genetic disease.17,18
Diagnosis criteria of Cowden Syndrome adapted from MESTER 201418
According to literature, in few cases, LDD could be associated with other malformation of the posterior fossa like Chiari malformation.4 Association with other lesions such as macrocephaly, polydactylies, multiple hemangioma, goiter, intestinal polyps was often observed.19 Diagnostic considerations or differential included glioma, subacute infarct, vascular malformation with cerebellar venous congestion, rhombencephalosynapsis, cerebellar dysplasia, medulloblastoma or, less likely, cerebellitis8,10
Current modes of treatment described in the literature consisted of medical (medications or radiotherapy) or surgical treatment, comprising of VP shunt or biopsy and lesion debulking4,20
Conclusion
LDD is a radiological curiosity due to its rarity, presentation, and location. The striped T2-weighted appearance of the cerebellum referred to as” tiger striping sign” gives it a typical characteristic recognizable by any radiologist proven for his diagnosis. The MRI features of LDD are almost unique and can be considered for diagnosis. The treatment can be either surgical or not. However, the definitive treatment of LDD is a surgical resection with decompression of the posterior fossa by total or subtotal tumor removal. Nowadays, Lhermitte–Duclos disease is regarded as a benign, treatable, and even curable condition.
Acknowledgments
The authors thank Billah Nabil Moatassim and Ittimade Nassar for their contribution and advice to succeed in this work.
Conflict of Interest
None declared.
References
- Lhermitte—Duclos disease in a young adult: rare entity. Iran J Pathol. 2013;8(03):194-198.
- [Google Scholar]
- Lhermitte-Duclos disease images in neurology Arq. Neuro-Psiquiatr. 2014;72(05):392-393.
- [Google Scholar]
- A case report of Lhermitte-Duclos disease revealed by psychiatric disturbances. Ann Gen Psychiatry. 2017;16:24.
- [Google Scholar]
- Lhermitte-Duclos disease: literature review and novel treatment strategy. Can J Neurol Sci. 1997;24(02):155-160.
- [Google Scholar]
- Case 144: dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) Radiology. 2009;251(01):298-303.
- [Google Scholar]
- Brain MRI features in Lhermitte-Duclos disease. Images in neurology Arq. Neuro-Psiquiatr. 2014;72(08):645.
- [Google Scholar]
- The Lhermitte-Duclos disease: a rare bilateral cerebellar location of a rare pathology. Pan Afr Med J. 2019;33:118.
- [Google Scholar]
- Acute presentation of Lhermitte-Duclos disease in adult patient in association with Cowden syndrome. Appl Radiol. 2016;45:28-31.
- [Google Scholar]
- Three adolescents with Lhermitte-Duclos disease. J Clin Neurosci. 2009;16(01):124-127.
- [Google Scholar]
- Assessing a dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) with 7T MR imaging. Korean J Radiol. 2010;11(02):244-248.
- [Google Scholar]
- MR imaging of Lhermitte-Duclos disease: a case report. AJNR Am J Neuroradiol. 1989;10(01):187-189.
- [Google Scholar]
- Lhermitte-Duclos disease associated with Cowden syndrome: a case report of a rare genetic hamartomatous disorder. Neurology. 2020;94:750.
- [Google Scholar]
- Bilateral recurrent dysplastic cerebellar gangliocytoma (Lhermitte-Duclos Disease) in Cowden syndrome: a case report and literature review. World Neurosurg. 2019;127:319-325.
- [Google Scholar]
- Lhermitte-Duclos disease. Images in neurology. Arq Neuropsquiatr. 2014;72(05):392-393.
- [Google Scholar]







