Translate this page into:
Phosphaturic mesenchymal tumor of the right thigh
* Corresponding author: Dr. Divya S, MD, Department of Radiation Oncology, Govt Medical College, Kozhikode, Uchimattom, Malloossery P.O., Kottayam, Kerala, India. divyamattom@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: S Divya, T Ajayakumar, Phosphaturic mesenchymal tumor of the right thigh. Asian J Oncol. 2025;11:10. doi: 10.25259/ASJO_62_2024
Abstract
Phosphaturic mesenchymal tumors (PMTs) are rare neoplasms often associated with tumor-induced osteomalacia (TIO). They present diagnostic challenges due to their rarity and nonspecific symptoms. A 44-year-old male presented with a one-year history of back pain, eight-month history of difficulty walking, and swelling over the right upper thigh. MRI of the dorsolumbar spine revealed subtle disc bulges and mild vertebral hypodensity. A bone scan suggested seronegative spondyloarthropathy. Positron emission tomography - Computer tomography (PET-CT) showed a somatostatin receptor-expressing lesion in the right proximal thigh. MRI confirmed a soft tissue lesion. Surgical excision and histopathology confirmed PMT. Preoperative serum phosphorus was low (1.6 mg/dL), and fibroblast growth factor 23 (FGF23) was elevated (352.4 pg/mL). Postoperative serum phosphorus normalized, and the patient received denosumab treatment. Follow-up over one year showed the patient remained asymptomatic with normal serum markers. This case highlights the importance of considering PMT in patients with persistent musculoskeletal pain and hypophosphatemia. Complete surgical excision is curative, and adjunct therapies like denosumab can be beneficial.
Keywords
Case report
Denosumab
Hypophosphatemia
Phosphaturic mesenchymal tumor
Tumor-induced osteomalacia
INTRODUCTION
Phosphaturic mesenchymal tumors (PMTs) are exceedingly rare, accounting for a minor fraction of mesenchymal tumors. They are often associated with tumor-induced osteomalacia (TIO), a paraneoplastic syndrome characterized by renal phosphate wasting, hypophosphatemia, and osteomalacia[1,2]. PMTs secrete fibroblast growth factor 23 (FGF23), which reduces renal phosphate reabsorption and suppresses 1α-hydroxylation of 25-hydroxyvitamin D, leading to hypophosphatemia and osteomalacia. Diagnosis can be challenging due to their nonspecific presentation and the rarity of the condition[3]. Patients often present with musculoskeletal pain, fractures, and muscle weakness, which can be mistaken for more common conditions such as osteoporosis or rheumatoid arthritis[4]. Imaging studies such as MRI and PET-CT are crucial for localising the tumor, while elevated FGF23 levels are a key diagnostic marker[1].
CASE REPORT
A 44-year-old male presented with chronic back pain for one year and progressive difficulty in walking and swelling over the right upper thigh for eight months. Physical examination revealed a firm, non-tender swelling measuring 2 × 1.2 cm in the medial aspect of the right proximal thigh. There were no palpable inguinal lymph nodes.
Diagnostic workup
MRI of the dorsolumbar spine showed subtle disc bulges at L4-L5 and L5-S1, causing anterior thecal sac indentation and mild diffuse T1 hypodensity of the vertebrae. A bone scan indicated asymmetrical active polyarthritis and bilateral sacroiliitis, suggesting seronegative spondyloarthropathy. A PET-CT scan with Ga DOTA-d-Phe1-Tyr3-octreotide (DOTATOC) demonstrated a somatostatin receptor-expressing lesion (2 x 1.2 cm) in the subcutaneous plane of the right proximal thigh and DOTATOC avid linear sclerosis in the bilateral sacral ala, indicating possible insufficiency fractures. MRI of the thigh confirmed a 22 × 12 × 10 mm soft tissue density lesion in the right proximal thigh’s subcutaneous location.
Laboratory findings
Preoperative serum markers showed hypophosphatemia (S. phosphorus: 1.6 mg/dL), normal calcium (S. calcium: 9.1 mg/dL), and elevated alkaline phosphatase (ALP: 127 IU/L). FGF23 levels were significantly elevated (352.4 pg/mL). Postoperative serum phosphorus levels normalized within 48 hours (6.6 mg/dL), and alkaline phosphatase (ALP) levels returned to baseline over three weeks.
Treatment
The patient underwent a wide excision of the tumor. Histopathological examination confirmed the diagnosis of PMT [Figures 1–3]. The tumor measured 2 cm with tumor-free margins. Postoperative serum phosphorus levels normalized (6.6 mg/dL), and ALP levels increased transiently (490 IU/L).
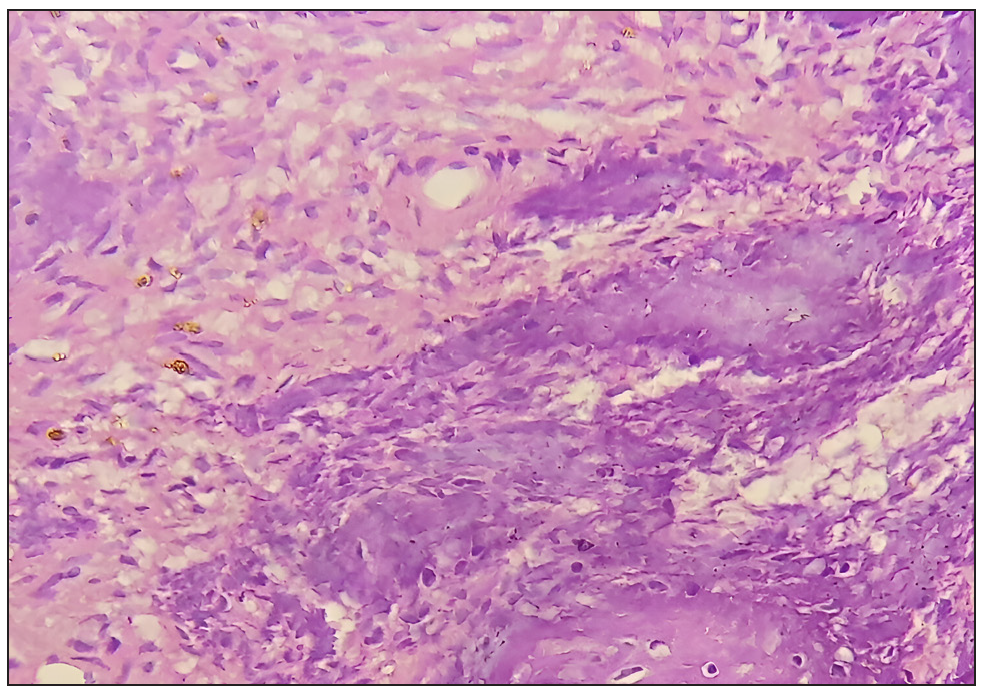
- High-power photomicrograph showing spindle cells in a myxoid stroma with thin-walled capillaries (Hematoxylin and Eosin, 400x).
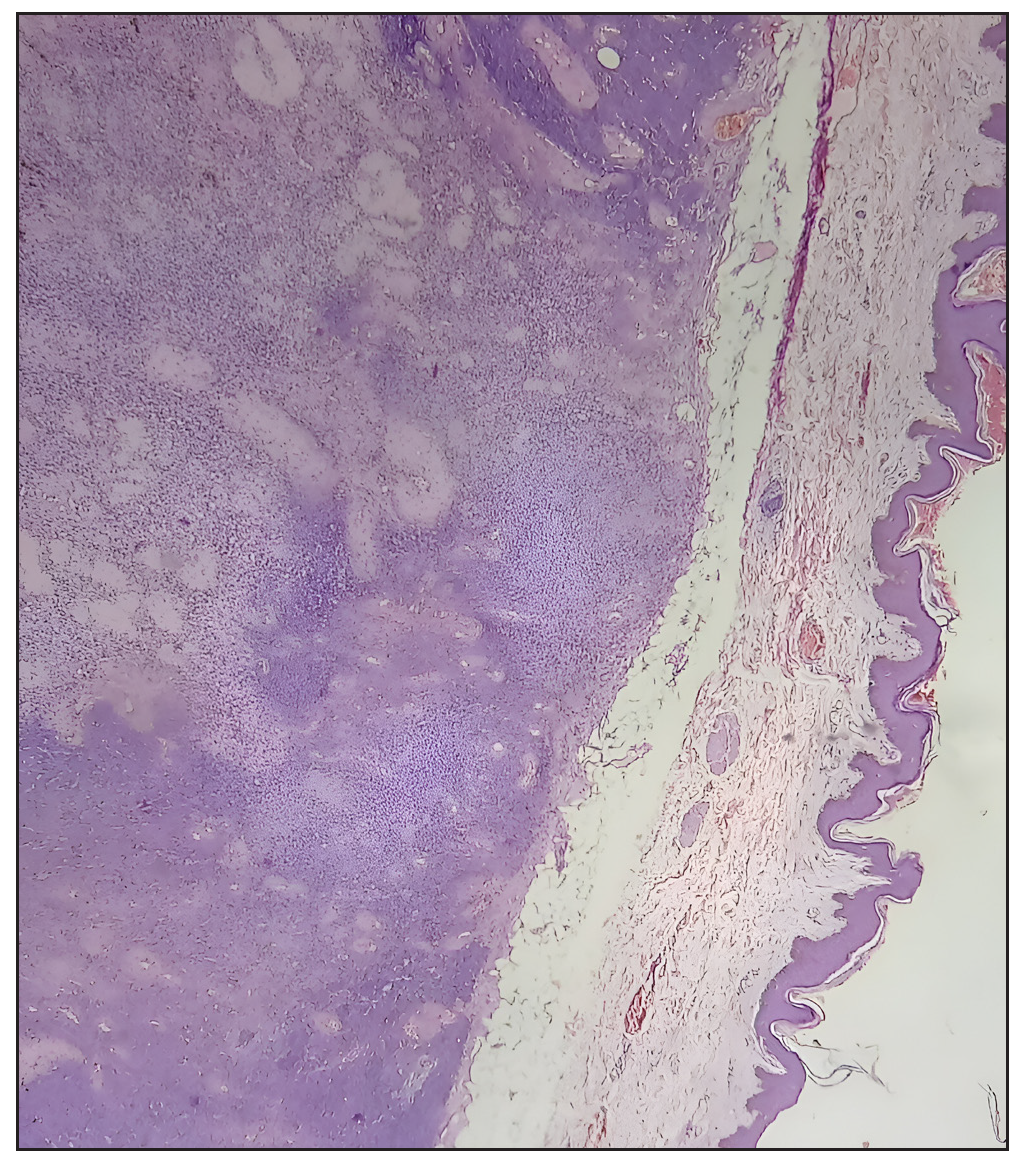
- Low-power photomicrograph demonstrating the tumor’s well-demarcated border (Hematoxylin and Eosin, 100x).
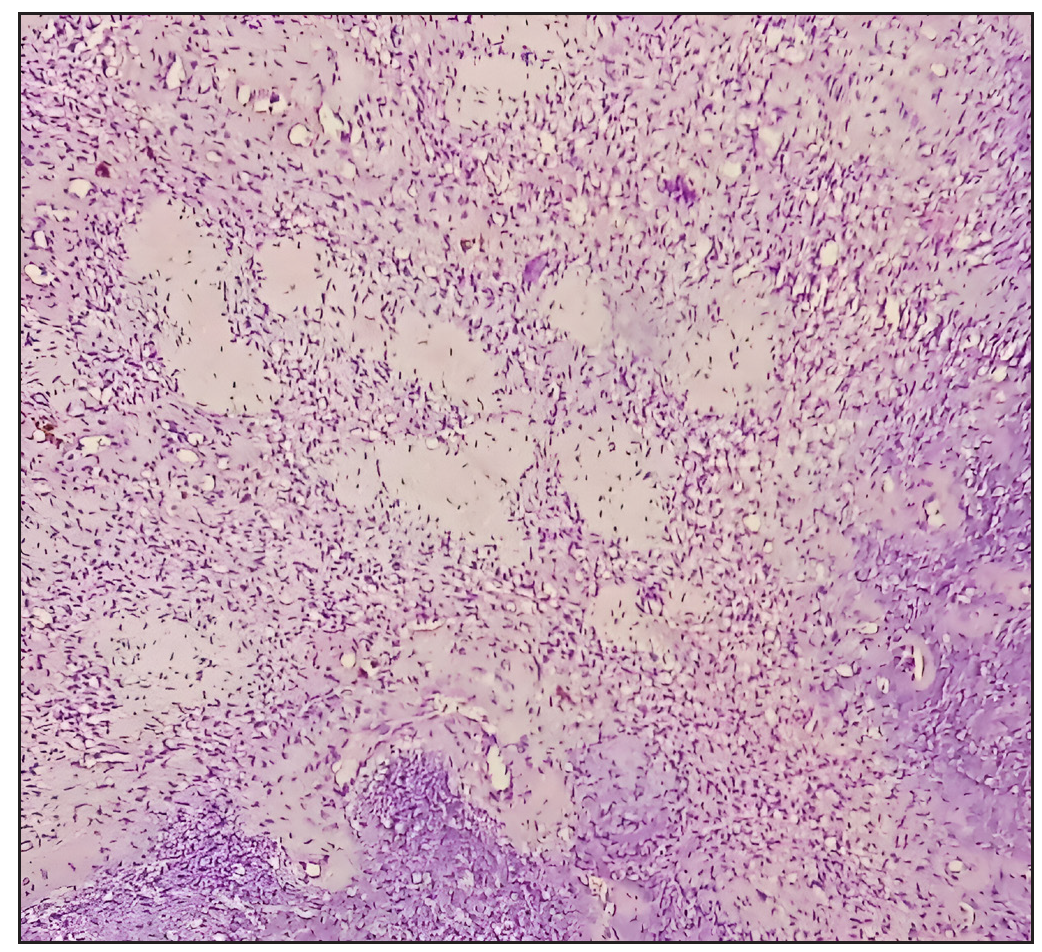
- Low-power photomicrograph highlighting capillary proliferation within the lesion (Hematoxylin and Eosin, 100x).
Follow-up and outcomes
The patient was treated with denosumab (120 mg s/c) for eight cycles over seven months, resulting in improved serum phosphorus levels. Due to financial constraints, denosumab was discontinued, and the patient has been on follow-up for the past year, remaining asymptomatic with normal serum markers.
DISCUSSION
PMTs are rare, and their diagnosis requires a high index of suspicion, particularly in patients presenting with unexplained hypophosphatemia and musculoskeletal symptoms[1]. Imaging studies such as MRI and PET-CT are crucial for localizing the tumor. Elevated FGF23 levels are a hallmark of TIO and guide the diagnosis[1]. Complete surgical excision of the tumor is curative, as demonstrated in this case.
Recent studies have highlighted clinical, radiological, and pathological features critical to diagnosing and managing PMTs. Benson et al. (2022) emphasized the role of advanced imaging modalities, particularly PET-CT with somatostatin receptor imaging, for accurate tumor localization. Their findings demonstrated that a systematic imaging approach enhances the identification of occult lesions, particularly in anatomically complex regions[5].
Histopathologically, PMTs exhibit a characteristic mix of spindle cells within a myxoid matrix and abundant vasculature. Folpe (2019) reviewed the morphologic diversity and molecular updates in PMTs, providing insights into the differential diagnosis, which often includes other soft tissue tumors. This study underscored the importance of correlating histopathological findings with clinical and imaging data for a definitive diagnosis[6].
Agaimy et al. (2017) expanded the spectrum of morphologic and immunophenotypic features of PMTs through an analysis of 22 cases, emphasizing the role of immunohistochemical markers such as FGF23. Their study also highlighted the molecular underpinnings of PMTs, contributing to a deeper understanding of their pathogenesis[7].
Surgical excision remains the cornerstone of PMT treatment, as it offers a potential cure by eliminating the source of FGF23 overproduction. A recent case report by J Sowell et al. (2021) reviewed surgical outcomes in elderly patients with PMT, demonstrating that complete resection leads to normalization of biochemical markers and symptomatic relief. However, achieving tumor-free margins is critical to avoid recurrence[8].
Adjunctive therapies like denosumab, which inhibits osteoclastic bone resorption, have shown promise in managing persistent hypophosphatemia post-surgery. This case supports the use of such therapies when surgical excision is incomplete or in cases of recurrent disease. Long-term follow-up is essential to monitor for recurrence and maintain normal serum markers.
Denosumab, a monoclonal antibody against receptor activator of nuclear factor - kappa B ligand (RANKL), has shown efficacy in treating PMT-associated TIO by reducing bone resorption and improving phosphate levels[9]. This case supports its use as an adjunct therapy, particularly when surgical resection is incomplete or in cases of recurrent disease. However, financial constraints can limit its long-term use.
Similar cases in the literature emphasize the importance of early recognition and treatment of PMTs to prevent long-term complications. Surgical excision remains the mainstay of treatment, with adjunctive therapies playing a supportive role.
By integrating recent advancements in diagnostic and therapeutic approaches, this report underscores the need for a multidisciplinary approach in managing PMTs, ensuring early detection and effective treatment to prevent long-term complications.
CONCLUSION
This case underscores the necessity of considering PMT in patients with unexplained musculoskeletal symptoms and hypophosphatemia. Early diagnosis and complete surgical excision are essential for a favorable outcome. Adjunctive treatments like denosumab can provide additional benefits, though accessibility may be an issue. Long-term follow-up is crucial to monitor for recurrence and maintain normal serum markers.
By reporting this rare case, we aim to enhance awareness and understanding of PMTs, facilitating timely diagnosis and treatment in future cases.
Author contributions
DS: Conceptualization, patient management, data collection, manuscript drafting, literature review, AT: Diagnostic workup interpretation, critical review of manuscript, final approval of the version to be published.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Octreotide therapy for tumor-induced osteomalacia. N Engl J Med. 2001;345:1883-8.
- [CrossRef] [PubMed] [Google Scholar]
- Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity. Am J Surg Pathol. 2004;28:1-30.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia. Nat Rev Dis Primers.. 2017;3:17044.
- [CrossRef] [PubMed] [Google Scholar]
- Phosphaturic mesenchymal tumor. AJNR Am J Neuroradiol. 2022;43:817-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phosphaturic mesenchymal tumors: A review and update. Semin Diagn Pathol. 2019;36:260-8.
- [CrossRef] [PubMed] [Google Scholar]
- Phosphaturic mesenchymal tumors. Am J Surg Pathol. 2017;41:1371-80.
- [CrossRef] [PubMed] [Google Scholar]
- Phosphaturic mesenchymal tumor: A case report and review of surgical outcomes in elderly patients. JAAD Case Rep. 2021;19:34-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phosphaturic mesenchymal tumors with or without phosphate metabolism derangements. Curr Oncol. 2023;30:7478-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








