Translate this page into:
Sarcoma of the thyroid co-occurrence with Carcinoma (Carcinosarcoma)
* Corresponding author: Dr. Priyadarshini Guha, Department of Pathology, Globe Health Care, Lucknow, Uttar Pradesh, India. drpriyadarshinigupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma K, Guha P, Agarwal A, Shukla N. Sarcoma of the thyroid co-occurrence with Carcinoma (Carcinosarcoma). Asian J Oncol. 2025;11:9. doi: 10.25259/ASJO_10_2024
Abstract
Thyroid carcinosarcoma (TC) is an aggressive biphasic malignancy. It has a high mortality rate even after the application of a Multi-disciplinary therapeutic approach. We studied a 63-year-old lady with neck swelling for 4 years with a rapid increase in size in 2 months. Fine Needle Aspiration Cytology (FNAC) of the thyroid nodule was reported as papillary thyroid carcinoma. Positron Emission Tomography and Computed Tomography (PETCT) showed a hypermetabolic lesion in the thyroid with a hypermetabolic lytic lesion in the 4th rib, suggestive of metastasis. Subsequently, patients underwent total thyroidectomy with central compartment clearance and left selective neck dissection. Histopathological evaluation and immunohistochemistry showed that spindle cells were positive for smooth muscle actin (SMA) and negative for Pan Cytokeratin (Pan CK), Carcinoembryonic Antigen (CEA), Paired-box-gene (PAX8), SRY-related HMG-box 10 (SOX10) and Desmin. Thyroid transcription factor 1 (TTF-1) was positive in follicular cells and Cytokeratin 19 (CK19) was focal positive. Hence, a diagnosis of Sarcoma with myoid differentiation and Follicular Carcinoma (carcinosarcoma) was made. Treatment of this disease remains not very clear. The method of management in is still Surgical removal of primary tumor.
Keywords
Anaplastic carcinoma
Carcinosarcoma
Follicular carcinoma
Immunohistochemistry
Thyroid
INTRODUCTION
Thyroid carcinosarcoma (TC) is an aggressive biphasic epithelial and mesenchymal malignancy. We have very limited information in literature about its aggressive nature and treatment strategies. Even after Multi-disciplinary therapeutic approach it has a high mortality rate. In this case report our aim was to discuss the role of detailed immunohistochemistry and role of surgeon in the management of such cases.
CASE REPORT
A 63-year-old lady reported to the Surgical Oncology unit of our hospital 3 years ago with a slow-growing neck swelling for a year. She had no other symptoms at that time.
Local examination of the neck revealed a firm, well-defined left thyroid nodule measuring 3 x 2 cm. There was no enlarged cervical lymph node. Routine blood investigations, including thyroid function tests, were normal. Ultrasound neck was done in our hospital and reported as (Thyroid Imaging Reporting and Data System-5) TIRADS 5 (Highly suspicious for malignancy). A subsequent thyroid scan detected a ‘cold’ nodular lesion. The fine needle aspiration cytology (FNAC) of the thyroid nodule was done outside and reported a papillary thyroid carcinoma. Based on these findings, the patient was scheduled for thyroidectomy, but the patient refused to undergo surgery due to her type II diabetes and Cardiac issues.
After 3 years, in September 2023, the patient revisited the Surgical Oncology unit of our hospital. The patient had a rapid increase in the size of thyroid nodules for two months along with dysphagia and dyspnea. Physical examination of the neck revealed a firm, well-defined left thyroid and isthmus nodule measuring 6 x 9 cm. Bilateral cervical lymph nodes were not palpable. Subsequently, PETCECT (positron emission tomography and computed tomography) showed a hypermetabolic lesion in the left lobe and isthmus of the thyroid with significant soft tissue component in 4th rib, suggestive of metastasis. Routine blood investigations, including thyroid function tests and 2D ECHO (two-dimensional echocardiography) Color Doppler were within normal range.
In October 2023, the patient underwent total thyroidectomy with central compartment clearance and left selective neck dissection (II to IV). The procedure was uneventful. The post-surgery investigation showed raised total leukocyte count (28000/dl) and raised TSH (Thyroid Stimulating Hormone) level. The patient was kept on medication and thyroxin support.
The specimen was sent to the histopathology department of our hospital. Gross examination revealed a poorly circumscribed fleshy to firm tumor, measuring 6.5 x 9 x 3.8 cm. Tumor involving left lobe thyroid and isthmus. The capsule was grossly intact [Figure 1]. Microscopy revealed tumors with two types of cellular populations. One comprised spindle-shaped tumor cells arranged in sheets and short fascicles with entrapped normal follicles. Individual cells have pleomorphic hyperchromatic nuclei containing prominent nucleoli and eosinophilic cytoplasm. Frequent mitoses and bizarre forms are seen [Figures 2 and 3], while the other comprised of tumor cells arranged predominantly in follicular pattern and exhibited nuclear enlargement, clearing with grooving in few [Figure 4]. Extensive areas of necrosis and calcification were noted in the capsule. Lymphovascular emboli are present [Figure 5]. Inferiorly capsule was involved in the tumor. All lymph nodes dissected were negative for metastasis (0/11).
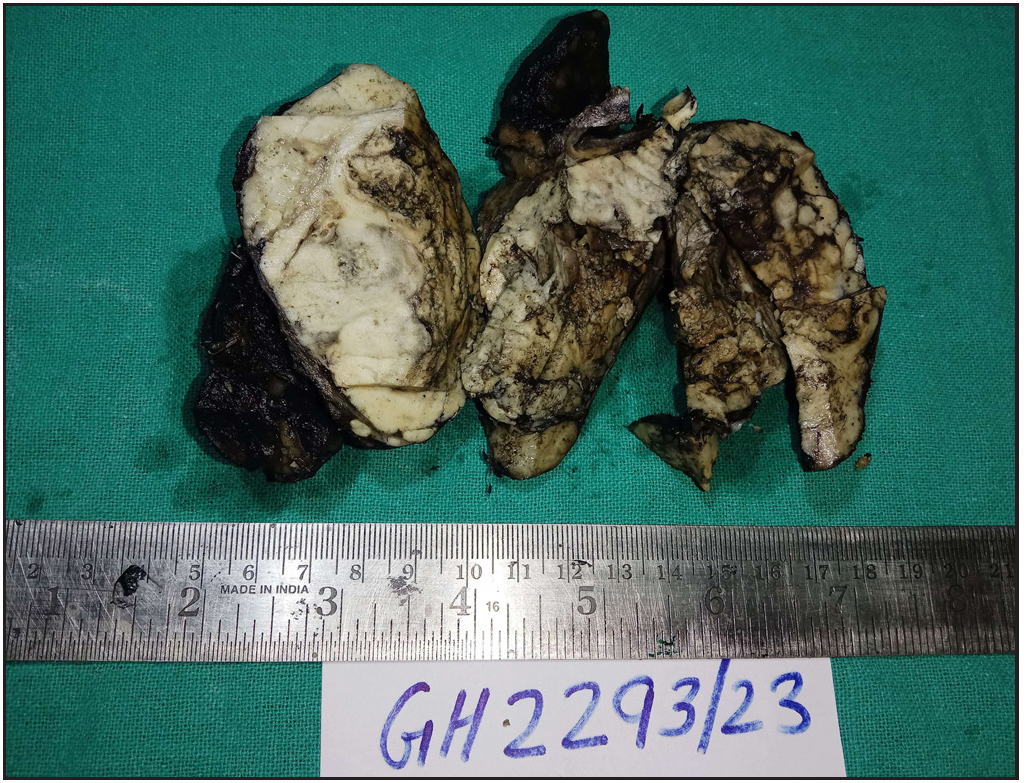
- Gross picture.
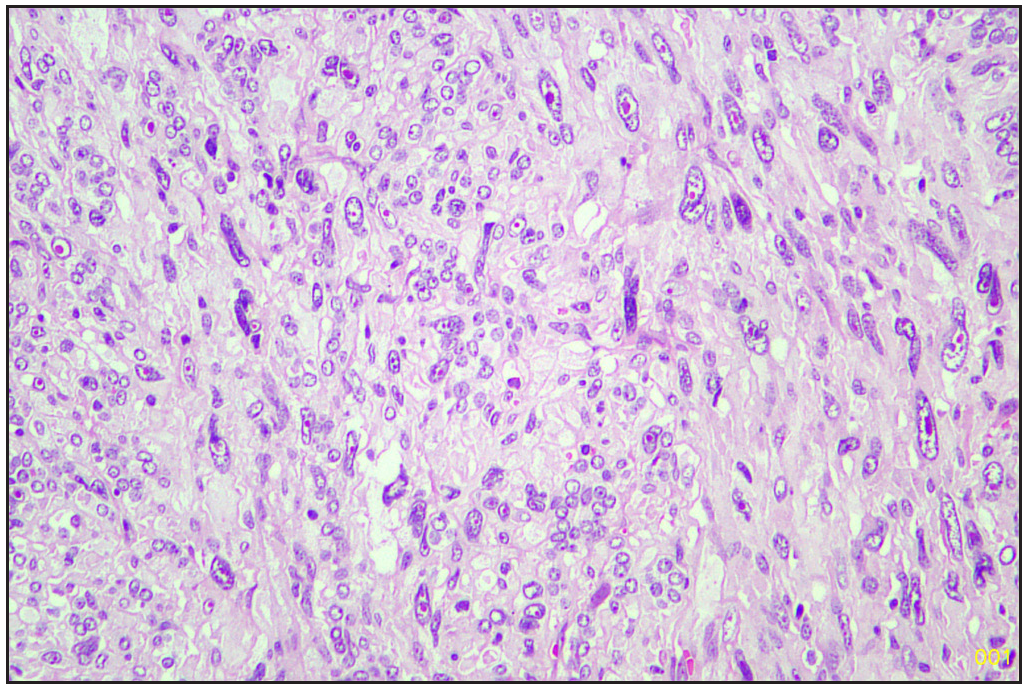
- Sarcomatous area (Hematoxylin and eosin, 20x).
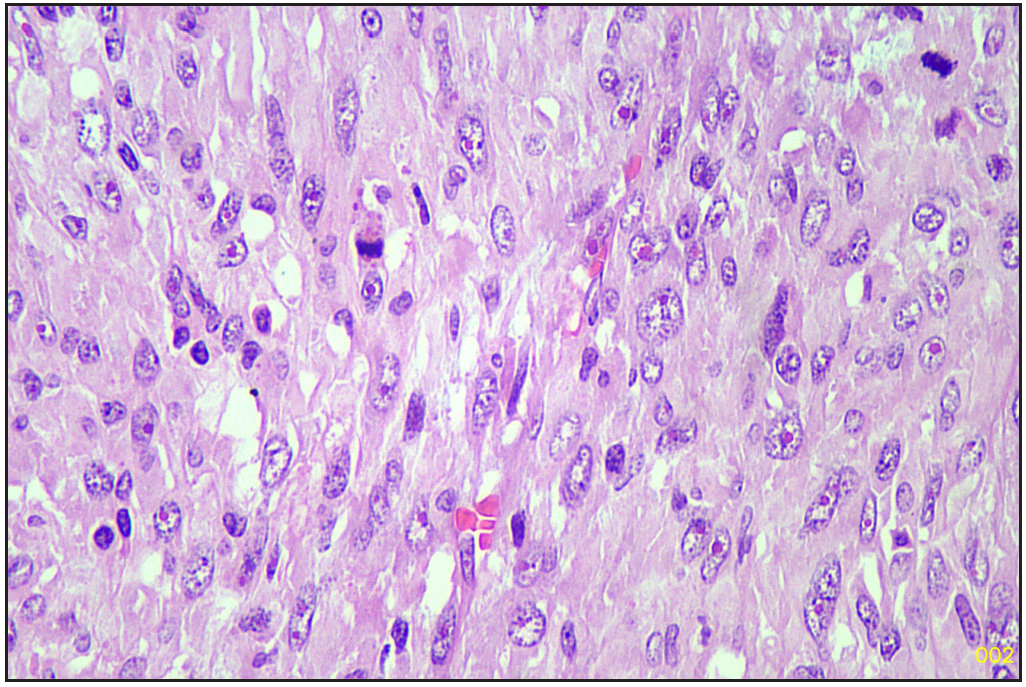
- Sarcomatous area (Hematoxylin and eosin, 40x).
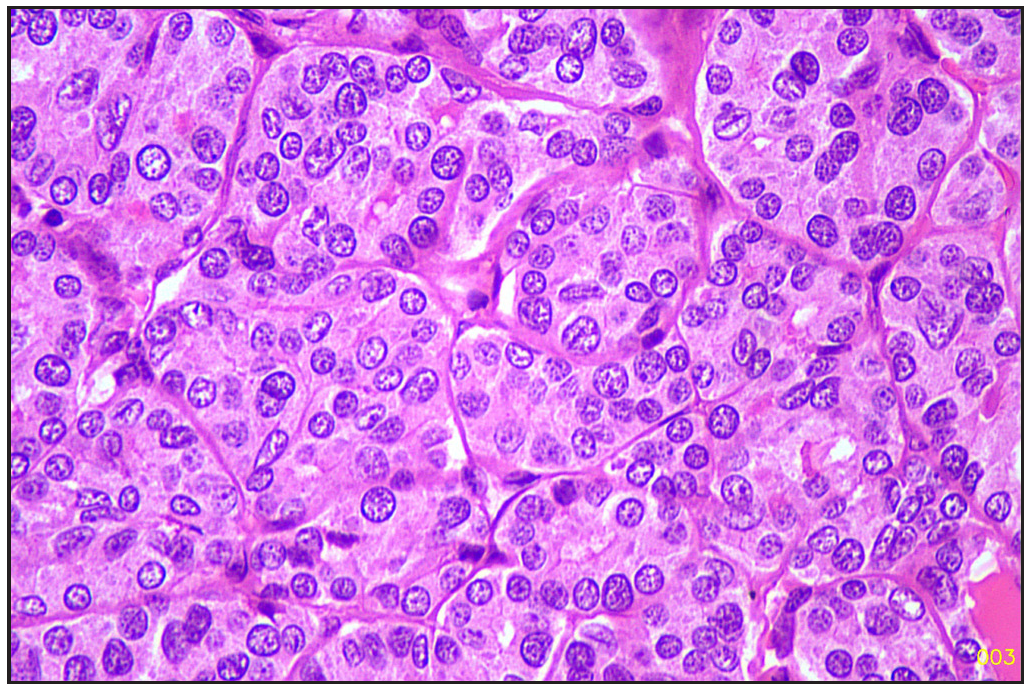
- Follicular area (Hematoxylin and eosin, 40x).
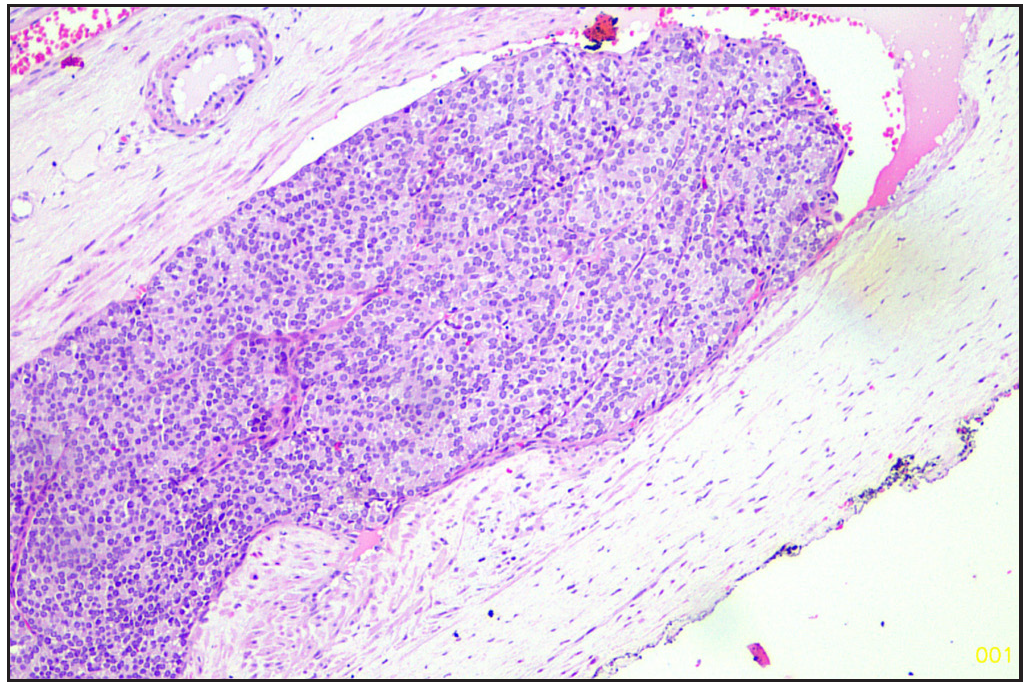
- Lymphovascular emboli (Hematoxylin and eosin, 20x).
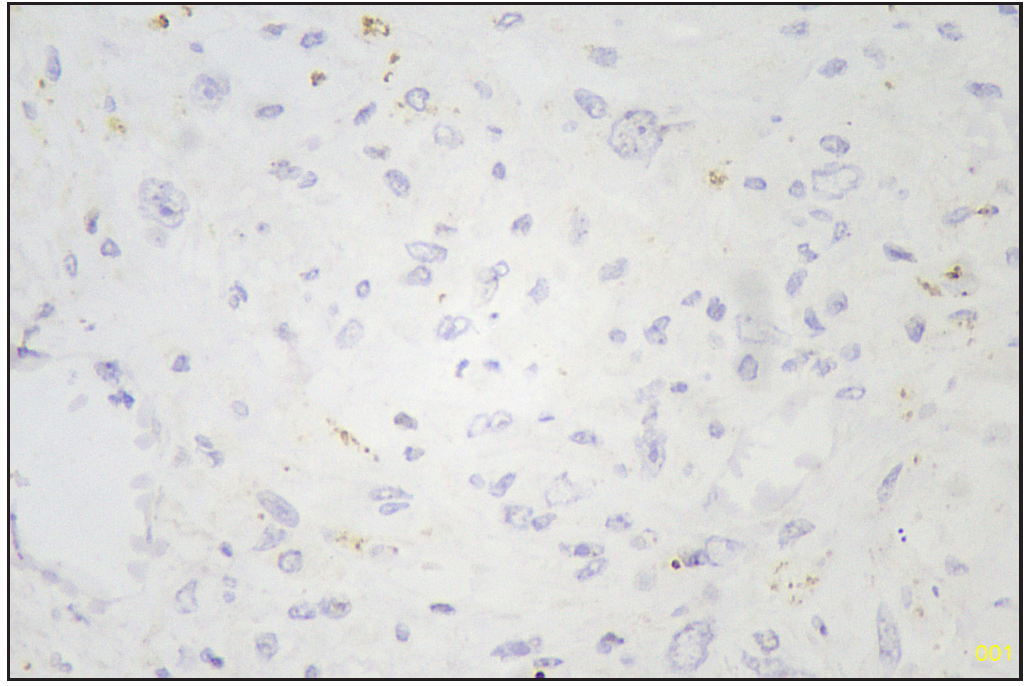
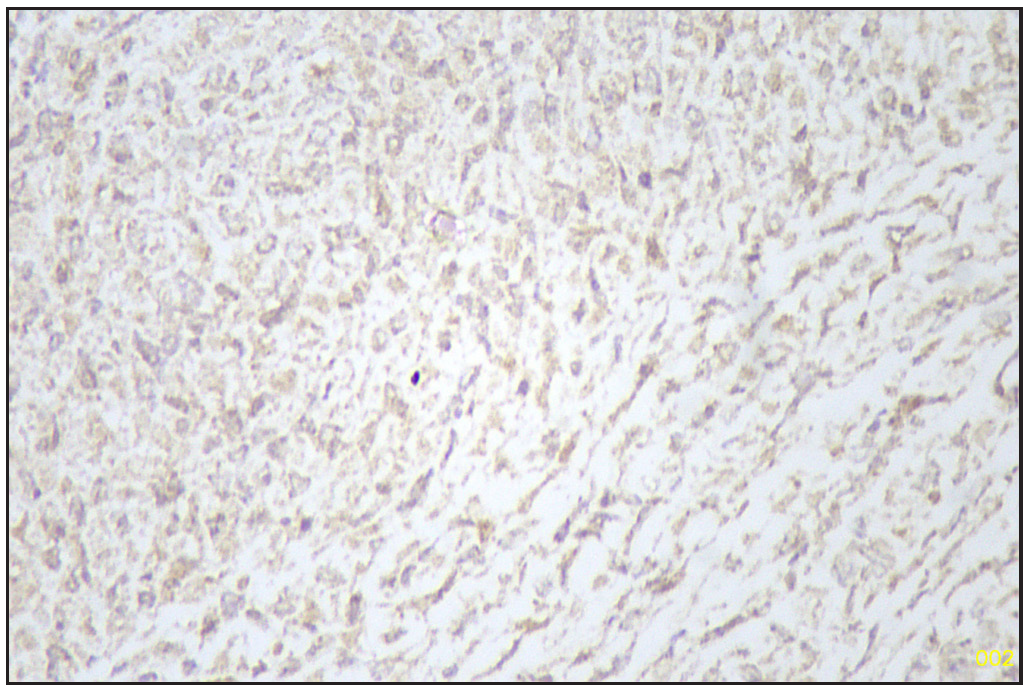
Based on morphology, a provisional diagnosis of Anaplastic carcinoma with a follicular variant of papillary thyroid carcinoma was made. Immunohistochemistry showed that the spindle cells were positive for Smooth Muscle Actin (SMA) [Figure 6] and negative for Pan Cytokeratin (Pan CK) [Figure 7], Carcinoembryonic Antigen (CEA), Paired-box-gene (Pax8) [Figure 8], SRY-related HMG-box 10 (SOX10) [Figure 9] and Desmin. Thyroid transcription factor 1 (TTF-1) [Figure 10] was positive in follicular cells, and Cytokeratin-19 (CK19) was focal positive. Hence, a diagnosis of sarcoma with myoid differentiation and follicular Carcinoma (carcinosarcoma) was made.
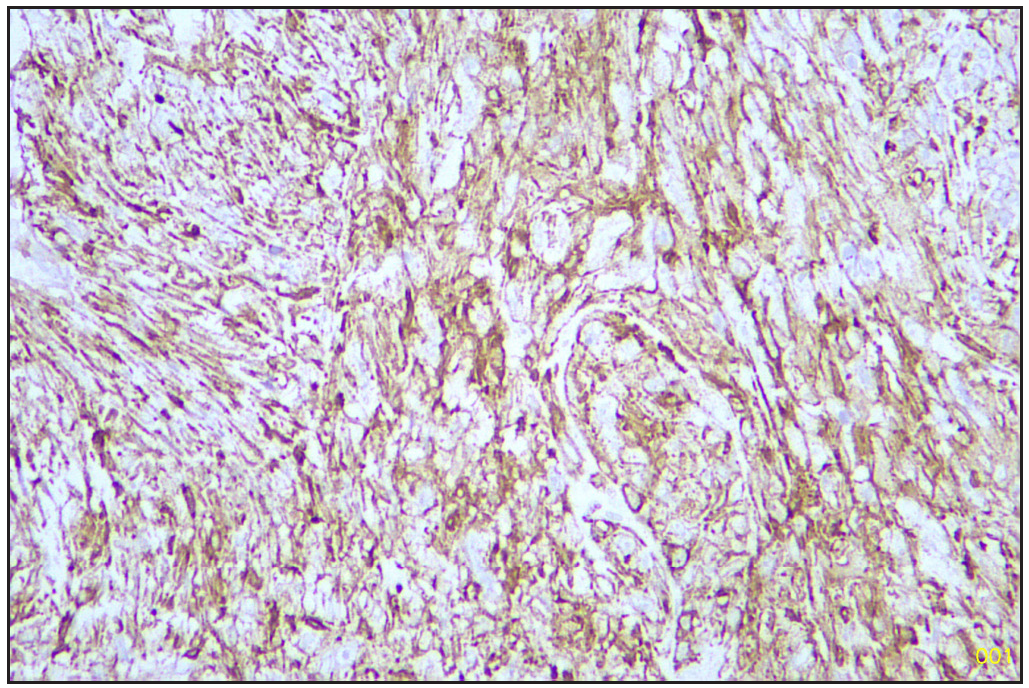
- SMA positive sarcomatous area (Immunohistochemistry stain, 40x). SMA: Smooth muscle actin.
- Pan CK negative sarcomatous area (Immunohistochemistry stain, 40x). CK: Pan-cytokeratin.
- Pax8 negative sarcomatous area (Immunohistochemistry stain, 40x).
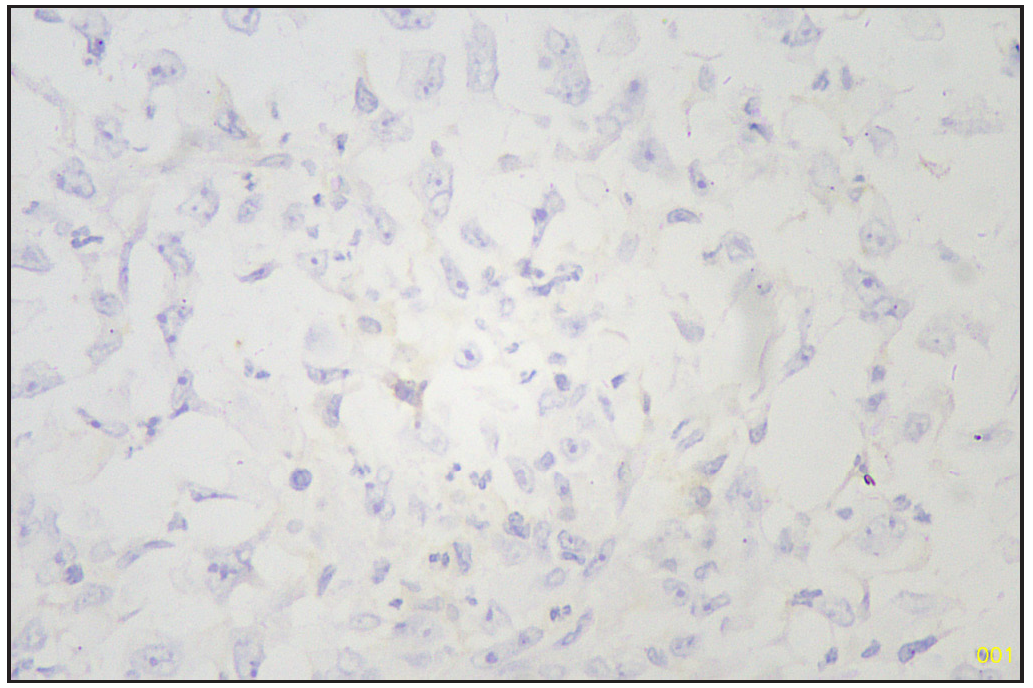
- SOX10 negative sarcomatous area (Immunohistochemistry stain, 40x). SOX10: Sry-related HMg-Box gene 10.
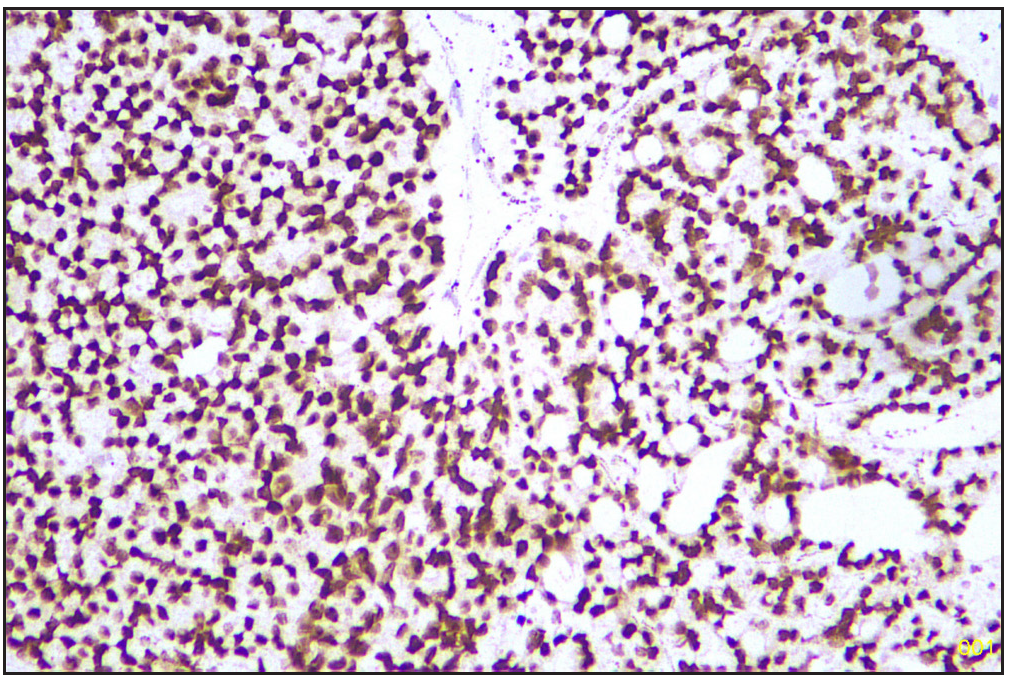
- TTF1 positive follicular area (Immunohistochemistry stain, 40x). TTF1: Thyroid transcription factor 1.
In November 2023, postoperative PETCECT showed a hypermetabolic lesion in the thyroid bed – suggestive of residual disease/recurrence of malignancy. Hypermetabolic pleural thickening, lung nodule with moderate pleural effusion along with hypermetabolic lytic lesion with significant soft tissue component in 4th rib, suggestive of metastasis.
After that, the patient underwent the first cycle of palliative chemotherapy. Carcinosarcoma was resistant to chemotherapy with minimal response. The patient succumbed to the disease one month later.
As this case is without identifiers, our institution does not require approval from Institutional Review Board (or its equivalent).
DISCUSSION
A literature search showed TC is an uncommon and aggressive tumor it comprises less than 0.1 percent of all thyroid malignancies.[1] This tumor is associated with a high fatality rate, survival is very short even after surgery or aggressive multimodal treatment.[2,3] Studies showed it commonly occurred in elderly women, as in our case.[4] In a review of 25 cases of carcinosarcoma, Agrawal et al. found sarcoma co-existed with follicular or papillary carcinoma, like in our case.[4] Studies showed that this tumor has a very poor prognosis, and death from the disease occurred within 2 to 26 months following diagnosis.[4] There is very limited knowledge about the pathogenesis and cause of poor prognosis. A recent study showed Carcinosarcoma thyroid associated with E1705K point mutation within the DICER1 gene and absence of RAS (Rat sarcoma), P53 (transformation-related protein 53), BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B mutations), which are seen in Anaplastic carcinoma.[5] Morphologically, it is very difficult to differentiate a sarcoma from an undifferentiated anaplastic carcinoma, and unfortunately, in 20% of anaplastic carcinoma cases, PanCK and Pax8 remain negative. However, in our case, most of the carcinoma markers like PanCK, CEA, Pax8, and TTF-1 were negative in sarcomatous areas, this favors sarcoma over anaplastic carcinoma.[6] Further mutation testing is needed. Recently World health organization (WHO) has not categorized carcinosarcoma as a distinct entity. It has been advocated that carcinosarcoma should be considered as a distinct entity from anaplastic carcinoma.[7]
As with our case, local recurrence with pulmonary or bony metastasis is common in carcinosarcoma due to its aggressive nature.[8]
The benefits of adjuvant treatment for the arrest of disease progression or to improve survival are not well-studied.[9,10]
CONCLUSION
To the best of our knowledge, very few cases an aggressive TC have been reported. Surgeons and Pathologists should be aware of the existence of this rare, prognostically poor disease. Detailed immunohistochemistry and molecular testing are crucial for diagnosis and to differentiate it from anaplastic carcinoma. The treatment of this disease remains not very clear. The method of management in metastatic disease is still surgical removal of the primary tumor.
Author contribution
KV: Contributed to the interpretation of the results. and writing the manuscript, PG: Writing the manuscript and correspondance, AA: Contributed to the final version of the manuscript, NSS: Contributed to the final version of the manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Thyroid carcinosarcoma, a rare and aggressive histotype: A case report. Ann Oncol. 2000;11:1497-9.
- [CrossRef] [PubMed] [Google Scholar]
- A rare malignant thyroid carcinosarcoma with aggressive behavior and DICER1 gene mutation: A case report with literature review. Thyroid Res. 2018;11
- [CrossRef] [Google Scholar]
- Carcinosarcoma of the thyroid gland. Case Rep Surg. 2015;2015:494383.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Carcinosarcoma thyroid: An unusual morphology with a review of the literature. South Asian J Cancer. 2013;2:226.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A rare malignant thyroid carcinosarcoma with aggressive behavior and DICER1 gene mutation: A case report with literature review. Thyroid Res. 2018;11:13044-018-0055-8.
- [CrossRef] [Google Scholar]
- Coexisting well-differentiated and anaplastic thyroid carcinoma in the same primary resection specimen: Immunophenotypic and genetic comparison of the two components in a consecutive series of 13 cases and a review of the literature. Virchows Arch. 2021;478:265-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Carcinome folliculaire avec dédifférenciation sarcomatoïde ou carcinosarcome de la glande thyroïde? [Follicular carcinoma with sarcomatoid dedifferentiation or thyroid gland carcinosarcoma?] Schweiz Med Wochenschr. 1979;109:86-92. French
- [PubMed] [Google Scholar]
- Thyroid carcinosarcoma, a rare and aggressive histotype: A case report. Ann Oncol. 2000;11:1497-9.
- [CrossRef] [PubMed] [Google Scholar]
- Management of thyroid carcinosarcoma. Surgery. 1997;122:548-52.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of anaplastic thyroid carcinoma Our experience. G Chir. 2010;31:282-5.
- [PubMed] [Google Scholar]







