Translate this page into:
When benign pretends to be malignant: A case report of giant cell tumor of the breast with review of literature
*Corresponding author: Dr. Rishi P Nair, MBBS, Department of Radiation Oncology, All India Institute of Medical Sciences, Basni, Jodhpur, India. rishiprem95@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aggarwal D, Nair RP, Kumar P, Devnani B. When benign pretends to be malignant: A case report of giant cell tumor of the breast with review of literature. Asian J Oncol. 2025;11:2. doi: 10.25259/ASJO_80_2024
Abstract
A giant cell tumor (GCT) of the breast is an uncommon intermediate-type soft tissue tumor with histological features resembling its osseous counterparts. To date, only 12 such cases have been documented. Our case involves a 58-year-old woman who initially presented with a breast lump progressively enlarging over six months. It was evaluated as a potential case of breast cancer. Mammography, biopsy, and, subsequently, positron emission tomography-computed tomography (PET-CT) were conducted for staging. The core biopsy specimen did not conclusively differentiate between the two suspected pathologies, i.e., GCT and Invasive breast cancer with osteoclast-like giant cells, although it was favoring the former. Following a multidisciplinary tumor board (MDTB) meeting, the patient underwent a total mastectomy and axillary lymph node dissection (TM+ALND). The large biopsy was suggestive of GCT of the breast.
We have conducted an exhaustive review of the literature on GCT of the breast, encompassing its presentation, radiological findings, differential diagnoses, and treatment modalities to formulate a structured approach for management. Additionally, we performed a statistical analysis of baseline characteristics, outcomes, and treatment modalities to succinctly summarize the current data on this subject.
Keywords
Breast tumor
Case report
Giant cell tumors
Osteoclast-like giant cells
Soft tissue sarcoma
INTRODUCTION
Giant cell tumors of soft tissue (GCT-ST) represent infrequent neoplasms characterized by a diminished propensity for malignancy. According to the Word health organization (WHO) classification of soft tissue tumors (2020), they are classified as intermediate-type tumors (rarely metastasizing) and giant cell tumors of soft parts.[1] These tumors were first reported in 1972 by Slam et al. and Guccion et al.[2,3] Originating from soft tissues, they are rich in giant osteoclast-like cells similar to conventional benign giant cell tumors of the bone (GCT-B).
GCT-STs predominantly occur in the soft tissues of the lower limbs, followed by the trunk and upper limbs.[4] GCT-ST of the breast is exceedingly rare, with only 12 cases reported thus far. There is no consensus on the treatment of breast GCT-ST, but all previous cases were managed with upfront surgical resection followed by observation. Adjuvant radiotherapy (RT) has been employed in many centers for cases of incomplete resection in GCT-ST and GCT-B. Incomplete surgical excision can lead to local recurrence, though there are no definitive guidelines for adequate margins. The average local recurrence rate for all GCT-STs is lower than for GCT-B, but the metastasis and mortality rates are higher. Therefore, close clinical follow-up is advised.[5]
In this article, we present a case report of an elderly woman with breast GCT-ST, accompanied by a literature review on all reported cases of such tumors to date. We will also discuss the available treatment modalities for GCT-STs.
CASE REPORT
A woman in her 50s with no known comorbidities presented to the outpatient department (OPD) with a complaint of a lump in her right breast for six months. The breast lump had an insidious onset, was gradually progressive, and was not associated with fever, pain, or discharge. There was no previous history of a lump in the opposite breast. The patient reported a history of a fall from a height, resulting in chest wall trauma eight months prior.
On examination, the patient was in fair general condition. Local examination revealed a 7 x 8 cm lump in the upper inner quadrant of the right breast, with no overlying exophytic growth. The lump was non-tender, hard in consistency, had irregular borders, and exhibited decreased mobility upon contraction of the underlying muscle. The contralateral breast and bilateral axillae appeared normal on palpation.
The presentation strongly indicated malignant breast cancer, necessitating further investigations for confirmation.
Investigations
Bilateral diagnostic digital breast tomosynthesis and ultrasound revealed a round, parallelly oriented, anechoic circumscribed mass with internal echogenic dependent contents, extensive dependent calcifications, and a few (2-3 in number) solid mural nodules (approximately 1.1x1.2 cm) with internal cysts showing vascularity in the inner quadrant of the right breast-BIRADS 4B. The left breast was BIRADS 1 (BIRADS-Breast imaging reporting and data system).
Histological analysis of an ultrasound-guided core needle biopsy revealed predominantly round to oval multinucleated osteoclastic cells arranged in sheets with atypical features. The pathology team suggested two differential diagnoses: either a GCT-ST or infiltrating breast carcinoma – no specific type (IBC-NST) with osteoclast-like giant cells. Endocrine receptors were negative. A repeat biopsy unfortunately, showed necrosed tissue. The biopsy was not repeated for a third time because the patient did not give consent for it, and also, in MDTB, it was discussed that repeating the biopsy would just delay the patient’s treatment.
Positron Emission Tomography- Computerized Tomography of the whole body (PET-CT-Scan) suggested a breast mass with Fluorodeoxyglucose (FDG) uptake in axillary level 1 lymph nodes and no other abnormal FDG-avid lesions in the rest of the body.
Differential diagnosis
To diagnose a GCT of the breast, one must consider differential diagnoses such as Phyllodes tumor, metaplastic carcinoma (IBC-NST with osteoclast-like giant cells), malignant fibrous histiocytoma, giant cell-rich leiomyosarcoma, and metastatic giant cell tumor of bone. Table 1 summarizes how clinical, pathological, radiological, and genetic findings contribute to reaching a definitive diagnosis.
| Differential diagnosis | Distinguishing feature |
|---|---|
| Phyllodes Tumor | Clinically- Rapidly growing, considerable size. Radiologically- solid cystic mass with posterior acoustic enhancement. |
| Metaplastic carcinoma (IBC-NST) | Pathologically- Epithelial markers |
| GCT rich leiomyosarcoma | Pathologically- Positive for SMA and desmin |
| Malignant fibrous histiocytoma | Pathologically- Marked cellular atypia and Pleomorphism |
| Metastatic GCT-B | Radiologically- Imaging for bone primary. Genetically- H3F3A mutation at G34 codon in tumour DNA sample |
IBC-NST: Invasive breast carcinoma- no specific type, SMA: Smooth muscle actin, GCT-B: Giant cell tumor of bone
Our case is the only one in which a PET-CT scan was performed. The objectives of conducting the PET-CT scan were:
-
1.
Metastatic workup, as primarily, the case was being dealt with as a cT3N1 IBC-NST with osteoclast-like giant cells.
-
2.
Excluding the possibility of an underlying primary GCT-B. It is crucial to rule out primary GCT-B in cases of GCT-ST, as the two entities share histological and immunohistochemical similarities.
There are two methods of differentiation between GCT-B and GCT-ST:[1] Radiological: Whole-body imaging is utilized to identify any primary bone lesions.[2] Genetic Analysis: Direct sequencing techniques on the deoxyribonucleic acid (DNA) amplified through polymerase chain reaction(PCR) from the tumor specimen to detect the H3F3A mutation at codon G34. This specific mutation is discernible in cases of GCT-B while notably absent in cases of GCT-ST.
Treatment
In our case, the initial biopsy indicated either GCT-ST of the breast or IBC-NST with osteoclast-like giant cells. The repeat biopsy revealed necrotic tissue. This presented significant challenges in determining the appropriate management strategy.
If the tumor were a GCT-ST of the breast, the optimal treatment would have been upfront surgical resection with clear margins. Conversely, if the diagnosis were IBC-NST, given the tumor’s classification as Stage 3A (cT3 cN1 M0), the preferred approach would have been neoadjuvant chemotherapy followed by surgery.
To balance the risk of overtreatment for GCT-ST and undertreatment for IBC-NST, we decided to perform an upfront total mastectomy along with axillary lymph node dissection (TM+ALND), thereby ensuring adequate surgical intervention for both potential diagnoses. The case was managed only after discussing it in Multi disciplinary tumor board (MDTB).
The large biopsy suggested a GCT-ST with negative resection margins and no lymph nodal involvement.
Follow-up
The patient was discussed in the multidisciplinary tumor board for further management. Due to the benign nature of the disease and complete resection (R0), the patient was scheduled for six-monthly follow-ups for local breast examination. Mammography and ultrasound of the abdomen were planned annually.
DISCUSSION
GCT-ST constitute a rare cohort of neoplasms frequently localized in the extremities. They have also been reported in the face, abdominal wall, shoulders, neck, and retroperitoneum, with the thigh being the most frequently reported site. GCT-STs of the breast are even rarer. According to our comprehensive literature review from PubMed, Scopus, and Google Scholar, only 12 GCT-ST of the breast have been reported to date.[6-17]
The clinical course of GCT-STs is highly unpredictable, necessitating proper management guidelines. As per literature, the standard treatment for GCT-ST is conservative surgical resection with tumor-free margins, ensuring a favorable prognosis and a low recurrence rate. There have been reports of local recurrence and lung metastases in patients with positive surgical margins.
Challenges arise when this neoplasm occurs in locations where surgical resection is anatomically unfeasible. In such situations, the following options can be considered:
-
1.
Bisphosphonates (Zoledronic and Pamidronic acid): In vitro studies have shown that bisphosphonates induce apoptosis in GCT-ST by causing post-translational effects on proteins involved in the Mevalonate pathway. There are no established guidelines for their use in GCT-B and GCT-ST. Mokrani et al. reported stable disease after eight monthly injections of zoledronic acid for a GCT-ST of the elbow.[18]
-
2.
Denosumab: This human monoclonal antibody targets RANK-L and induces apoptosis in osteoclastic giant cells. The National Comprehensive Cancer Network (NCCN) guidelines recommend it for non-resectable GCT-B. Based on its mechanism of action, Denosumab may be tried in GCT-ST, although there are no specific guidelines except for one case report by Nepucpan et al. where zoledronic acid and Denosumab were used for an unresectable GCT-ST of the nasopharynx, resulting in stable disease after six months.[19]
-
3.
Interferon alfa and pegylated interferon alpha: These anti-angiogenic agents are helpful due to the highly vascularized nature of GCT-ST. Partial responses have been observed in patients treated with these agents.
-
4.
Radiation therapy: The role of radiotherapy in patients with incomplete resection or inoperable GCT-ST remains controversial. In cases with positive surgical margins, adjuvant radiotherapy has been tried with varying success rates. The possibility of post-radiation sarcomatous changes cannot be ruled out in such patients. Hence, this treatment should be considered with caution.
-
5.
Angioembolization has also been used to downsize the tumor in GCT-STs, unresectable at the outset. However, it has its limitations, such as the need for a sufficiently sized feeder vessel, which, if absent, makes the procedure futile.
To date, only 12 cases of GCT-ST of the breast have been reported.[6-17] Among these, 11 cases involve female patients, with a single case reported in a male patient.
The median age of disease presentation in the existing literature is 59 years, the mean age is 57.6 ± 11.14 years, ranging from 36 to 75 years. Eight of these 13 cases (61.5%) originate from Asia, suggesting a potential predilection for the disease in this region, although further prospective data is needed. All patients presented with a breast lump, with pain reported in four cases and bloody discharge in one case. There was a history of chest trauma in two cases—one previously reported and one in our case [Table 2].
| S.N | Author new |
Demographic area |
Age/gender | Presentation and symptom | Trauma | Size (cm) | Side | Quadrant | Characteristic |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fukunaga et al. (2002)[6] | Tokyo, Japan | 68/F | Incidentally detected a small mass. | No | 2.5 | Right | Lower outer | Not mentioned |
| 2 | Shousha et al. (2004)[7] | London, UK | 59/F | Hard breast lump for two years, which was alarmingly increasing in size. | No | 4 | Left | Not mentioned | Hard, irregular, painless with fixation to pectoralis. |
| 3 | May et al. (2007)[8] | Texas, USA | 60/F | Self-detected lump. | Yes | 3 | Left | Lower inner | Hard, regular, painless. |
| 4 | Romics et al (2009)[9] | Glasgow, UK | 50/F | Discrete swelling. | No | 2.5 | Left | Upper outer | Not mentioned |
| 5 | Gaspar et al. (2017)[10] | Chandigarh, India | 36/F | Rapidly increasing left breast lump over 1.5 years. | No | 7 | Right | Upper outer | Hard, Phyllode’s tumour was suspected. |
| 6 | Sawa et al. (2019)[11] | Ibaraki, Japan | 45/F | Rapidly enlarging lump with pain and bloody nipple discharge. | No | 5 | Left | Center | Hard. |
| 7 | Terada et al. (2019)[12] | Aichi, Japan | 74/F | Noticed a tender lump | No | 2.5 | Right | Upper outer | Hard, tender with palpable lymph node in the right axilla. |
| 8 | Luangxay et al. (2020)[13] | Tokyo, Japan | 59/F | Pain in the left breast | No | 2.5 | Left | Upper outer | Rubbery non-tender mass fixed to the skin. |
| 9 | Zhang et al. (2021)[14] | Beijing, China | 65/F | Self-detected lump. | No | 2 | Left | Upper inner | Firm, non-tender. |
| 10 | Novrial et al. (2022)[15] | Central Java, Indonesia | 45/F | Self-detected lump. | No | 2 | Left | Upper outer | Firm, non-tender, well defined. |
| 11 | Suleman et al. (2022)[16] | Pretoria, South Africa | 58/F | Self-detected lump. | No | 2 | Left | Upper outer | Firm, non-tender, well defined. Phyllode’s tumour was suspected. |
| 12 | Lucas et al. (1981)[17] | Columbus, Ohio. | 72/M | Self-detected lump with dull pain. | No | 13 | Right | Not mentioned | Non-tender, non-fixed, rubbery with an ecchymotic patch on the skin. |
| 13 | Present case | Rajasthan, India | 58/F | Self-detected lump | Yes | 8 | Right | Upper inner | Hard lump with no overlying exophytic growth. |
UK: United Kingdom, USA: United states of America.
The mean tumor size at presentation is 4.3 ± 3.26 cm, with a median of 2.5 cm, ranging from 2 to 13 cm. Most documented cases involve the upper outer quadrant of the left breast [Figure 1].
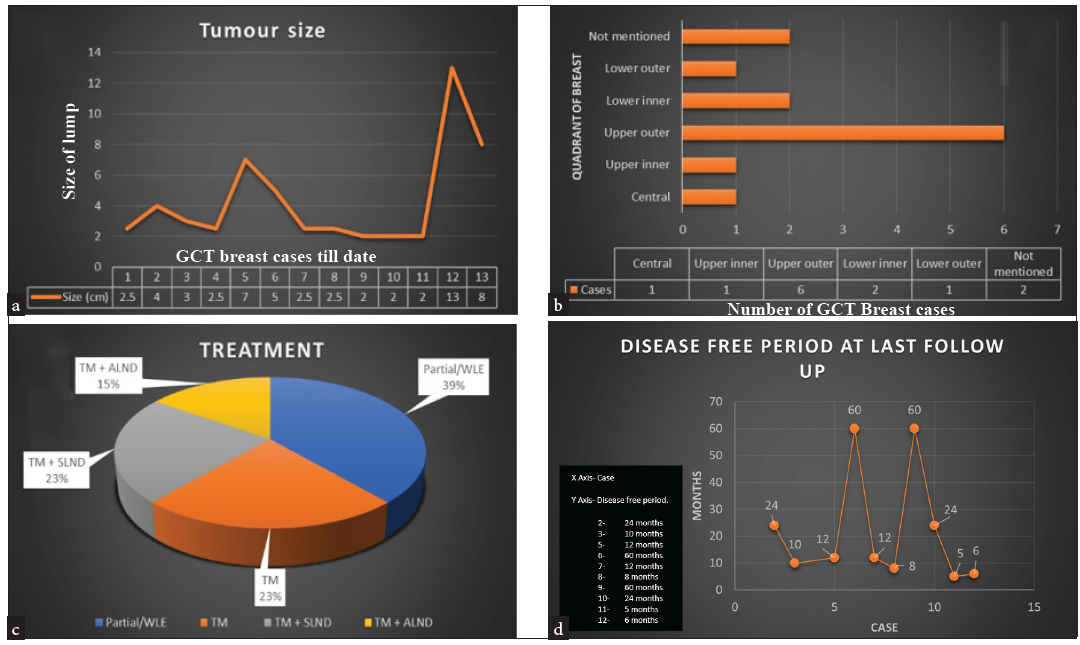
- Analysis of cases reported to date: (a) The trend in tumour size at presentation. (b) The quadrant of tumour presentation. (c) The treatments received in cases across the literature, including wide local excision (WLE), total mastectomy (TM), total mastectomy with sentinel lymph node dissection (TM+SLND), and total mastectomy with axillary lymph node dissection (TM+ALND). (d) The disease-free period at the last follow-up in each case. GCT: Giant cell tumor.
The radiological features of GCT-STs in the breast remain inadequately delineated owing to their uncommon occurrence. However, based on the reported cases and our findings, it can be inferred that GCT-ST typically presents as a well-demarcated solid and cystic mass with mixed echogenicity on breast sonography, an irregular mass lesion with lobulated margins on mammography, and a hyperintense lobulated irregular mass on T2-weighted MRI of the breast.
Out of the 12 reported cases of GCT-ST of the breast, only one underwent total mastectomy and axillary lymph node dissection (TM+ALND). Our case represents the second instance where axillary lymph node dissection was performed.
The patient discussed by May et al.[8] experienced recurrence six months after partial excision; a total mastectomy was subsequently performed, and histopathology confirmed GCT-ST. Unfortunately, the patient developed lung metastasis two months post-mastectomy and died within ten months of the initial presentation.
In a similar scenario, Zhang et al.[14] also performed a partial excision. The patient developed lung metastases a year later and underwent metastasectomy. Histopathology confirmed that the metastasis originated from the breast. Referring to the treatment protocol for soft tissue sarcomas, they administered ifosfamide and epirubicin for four cycles in an adjuvant setting.
Suleman et al.[16] performed a total mastectomy without ALND in their case. The patient experienced recurrence five months later, with biopsy findings indicative of poorly differentiated malignancy with osteoclast-like giant cells. They planned radiotherapy and Adriamycin-based chemotherapy for their patient.
The main issue with the management of our case was the direct implementation of ALND to prevent under-treatment of the invasive breast carcinoma. We propose that, in the future, the classical algorithm of axillary lymph node sampling and sentinel lymph node biopsy should be followed, even if the diagnosis is pre-operatively confirmed as a GCT-ST of the breast.
In future cases, we recommend an approach involving upfront surgical resection, whether through mastectomy or breast-conserving surgery (BCS). However, axillary lymph nodes should be carefully addressed using the principles applied in the management of invasive breast carcinoma, but not necessarily through immediate ALND. Instead, a more selective approach, such as sentinel lymph node biopsy or axillary node sampling, should be prioritized to avoid unnecessary surgical procedures while still ensuring a thorough evaluation of nodal involvement.
CONCLUSION
-
1.
Despite the rarity of the breast’s GCT-STs, pathologists, surgical oncologists, medical oncologists, and radiation oncologists must retain them within their diagnostic purview.
-
2.
Upon diagnosis of a GCT-ST of the breast, it is imperative that the case undergoes deliberation within a multidisciplinary tumor board, with treatment strategies meticulously tailored to the individual patient.
-
3.
The therapeutic regimen should primarily target surgical excision of the tumor with due attention to achieving adequate margins. Axillary lymph nodes must be addressed according to the principles applied for invasive breast carcinoma management. The consideration of radiotherapy should be approached cautiously, given the attendant risk of sarcomatous transformation.
-
4.
Presently available literature does not furnish substantiated evidence advocating for the utility of adjunctive treatments.
-
5.
Consistent with the best practices, regular follow-up appointments with the patient, scheduled for six-month intervals, are advisable.
Acknowledgment
We want to express our sincere gratitude to all those who contributed to the successful completion of this case report. Special appreciation is extended to the pathologists and radiologists for their insightful diagnostic contributions. We would like to thank the patient for their consent and trust in sharing their case, which has provided invaluable learning for both the medical community and us.
Author contributions
DA and RPN: Responsible for drafting the manuscript, conducting statistical analyses, and preparing tables and figures, PK and BD: Provided critical revisions to the manuscript and approved the final version for submission.
Ethical approval
The Institutional Review Board has waived ethical approval for this study
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- The 2020 WHO Classification of soft tissue tumors: News and perspectives. Pathologica. 2020;113:70-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Malignant giant cell tumor of soft parts. An analysis of 32 cases. Cancer. 1972;29:1518-29.
- [CrossRef] [Google Scholar]
- Giant cell tumor of soft tissue: A rare entity. Orthopedics. 2019;42:e364-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary giant cell tumor of soft tissues: A study of 22 cases. Am J Surg Pathol. 2000;24:248.
- [CrossRef] [PubMed] [Google Scholar]
- Chest wall tumors presenting as breast lumps. Breast J. 2004;10:150-3.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of soft tissue arising in breast. Ann Diagn Pathol. 2007;11:345-9.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoclast-like giant cell tumor arising in the soft tissue of the breast: report of a case. Surg Today. 2009;39:48-51.
- [CrossRef] [PubMed] [Google Scholar]
- Primary giant cell tumor of the female breast: A diagnostic red herring with therapeutic implications. APMIS. 2017;125:32-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative diagnosis of a giant cell tumor of soft tissue arising from the breast by ultrasound-guided core needle biopsy. J Med Ultrasonics. 2019;46:257-61.
- [CrossRef] [Google Scholar]
- A case of giant cell tumor of the breast, clinically suspected as malignant breast tumor. Surg Case Rep. 2019;5:77.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of soft tissue of the breast: Case report with H3F3A mutation analysis and review of the literature. Pathology - Research and Practice.. 2020;216:152750.
- [CrossRef] [PubMed] [Google Scholar]
- Primary giant cell tumor of the breast with pulmonary metastasis: A case report and review of the literature. Front Oncol. 2021;11 [accessed on 2024 May 11]. Available from: https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2021.638237/full
- [CrossRef] [Google Scholar]
- Primary giant cell tumor of soft tissue in the female breast. Jurnal Profesi Medika: Jurnal Kedokteran dan Kesehatan. 2022;16 [accessed on 2024 May 11]. Available from: https://ejournal.upnvj.ac.id/JPM/article/view/3204
- [CrossRef] [Google Scholar]
- Primary giant cell tumor of the breast with recurrence: A rare case report. SA Journal of Radiology.. 2022;26 [accessed on 2024 May 11]. Available from: https://www.ajol.info/index.php/sajr/article/view/241446
- [Google Scholar]
- Unusual giant cell tumor arising in a male breast. Human Pathology. 1981;12:840-4.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of soft tissues: A case report and review of literature. J Cancer Res Ther. 2017;09 [accessed on 2024 May 29]. Available from: https://cir.nii.ac.jp/crid/1360011143569178240
- [CrossRef] [Google Scholar]
- Giant cell tumor of soft tissue of the nasopharynx: A case report. Cancer Treat Res Commun. 2020;23:100171.
- [CrossRef] [PubMed] [Google Scholar]








