Translate this page into:
Changes in Plasma Beta-Endorphin Levels in Stage III–IV Nasopharyngeal Carcinoma Patients Post World Health Organization 3-Step Analgesic Ladder Therapy
Address for correspondence Rizka Fathoni Perdana, Department of Otorhinolaryngology Head and Neck Surgery, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Jl. Mayjend Prof. Dr. Moestopo No. 6-8, Mojo, Gubeng, Surabaya, East Java 60286, Indonesia. rizka-f-p@fk.unair.ac.id
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction Nasopharyngeal carcinoma (NPC) is the most common malignancy in the field of otorhinolaryngology, and chronic pain is identical with this malignancy. Pain therapy according to World Health Organization (WHO) recommendations is WHO 3-step analgesic ladder. Pain is subjective and related to the function of beta-endorphin hormone.
Objective Analyzing the relationship between the degree of pain and plasma endorphin levels in stage III–IV NPC patients before and after the administration of WHO 3-step analgesic ladder.
Materials and Methods The study design used pretest and posttest without control design. Participants were given WHO 3-step analgesic ladder therapy for 3 days. The participants then rated the pain scale using the visual analog scale (VAS) and plasma beta-endorphin levels in venous blood. The statistical test used the dependent t-test, Wilcoxon test, and Spearman test with p < 0.05, confidence interval: 95%.
Results There were 14 stage-III NPC patients with moderate pain (78.57%) and 31 stage-IV NPC participants had moderate pain (83.87%; p = 0.071). The VAS value in the moderate pain group before and after therapy was 82.22% and 66.67%, respectively (p < 0.001). The values of plasma beta-endorphin levels before and after therapy were 74.89 ± 69.12 and 72.49 ± 75.53 pg/mL, respectively (p = 0.647). Plasma beta-endorphin levels were −19.20 ± 37.72 pg/mL (mild pain), −4.76 ± 35.30 pg/mL (moderate pain), and −21.67 ± 6.27 pg/mL (severe pain; p = 0.717).
Conclusion Pain levels in advanced NPC patients have decreased after the therapy, but plasma beta-endorphin levels have no significant difference.
Keywords
beta-endorphin
pain
nasopharyngeal carcinoma
analgesic
Introduction
The World Health Organization (WHO) reports that there are approximately 7 million new cancer patients each year. Nasopharyngeal carcinoma (NPC) is still the most malignant tumor in the field of otorhinolaryngology head and neck surgery, with a frequency >50% of all head and neck malignancies. In Indonesia, the average prevalence recorded is 6.2/100,000, with 13,000 new NPC cases every year, but on the contrary, there are still limited documentations regarding this disease.1 Nearly 89% of patients seeking treatment at hospital came with a condition of an advanced-stage NPC.2 The main complaint of NPC patients is chronic pain3 and as many as 60 to 100% of NPC patients with mild-to-severe pain had difficulties in performing their daily activities.4
The most effective management of cancer pain referring to several studies is WHO 3-step analgesic ladder.5,6,7 The WHO 3-step analgesic ladder, which is a tiered analgetic administration, consists of three stages, namely the initial analgesic stage that administers a nonopioid analgesic medication, the second stage is a weak opioid and nonopioid analgesic, and the third stage is the administration of a strong opioid and nonopioid analgesics.7,8 The most commonly used evaluation method for the 3-step analgesic ladder is the visual analog scale (VAS). It is an easy-to-use assessment tool in evaluating the pain of NPC patients. However, in its implementation, VAS has limitations in several conditions, including patients with decreased consciousness or patients who are unconscious, patients with cognitive limitations, elderly patients, and pediatric patients.9 Therefore, a more objective assessment of the pain scale is needed to assess the pain scale of NPC patients before and after obtaining the WHO 3-step analgesic ladder. Beta-endorphin is an indicator that can be used to evaluate the pain intensity of patients.10,11
Beta-endorphins are closely related to pain, especially in their function in the analgesic system. Beta-endorphins are endogenous morphine which play a role in endogenous pain relief.10,11 In the blood vessels, the secreted beta-endorphins will quickly disappear due to metabolic processes. The working duration of secreted beta-endorphins is still unclear. The research on how beta-endorphins can relieve pain is still limited, especially the measurement of beta-endorphin levels in the body.12
Our hospital is one of the main referral hospitals with complete facilities located in the city of Surabaya, Indonesia. Surabaya is the second largest city after Jakarta, Indonesia. The number of NPC patients undergoing treatment at hospital is increasing every year. Research related to the activation time of beta-endorphin in the body after the administration of WHO 3-step analgesic ladder in NPC patients is still very limited. Based on the description above, it is necessary to examine changes in beta-endorphin levels of NPC patients after the administration of analgesia drugs according to WHO 3-step analgesic ladder. The aim of this study was to analyze the relationship between the degree of pain and plasma endorphin levels in stage III–IV NPC patients before and after the administration of WHO 3-step analgesic ladder.
Materials and Methods
Participants in this study included advanced-stage NPC patients (stage III and IV) who were currently undergoing treatment at hospital. Participants must meet the following inclusion criteria: (1) patients diagnosed with NPC clinically, anatomically, and histopathologically,13,14 (2) patients aged 20 to 75 years; (3) patients experiencing pain that can be assessed using VAS; and (4) patients not receiving pain treatment. Participant exclusion criteria included patients having comorbidities that caused pain (such as gout arthritis), metabolic diseases (such as diabetes mellitus, hepatitis, and kidney disease), patients with weak physical conditions (Karnofsky performance scale < 70%), and patients with communication problem. Participants received an explanation related to the study, especially the rights and obligations of the participant during the study before filling out the consent form.
The design of this study used a pretest and posttest without control design. The study was carried out in the period January to December 2012. The number of participants was 45, which were selected using a consecutive sampling method (Fig. 1). An ethics test was conducted prior to the study by the Ethics Committee at the hospital (number: 04/Panke.KKE/I/2011). The procedure of this study included evaluation of participant's characteristics, pain scale, and plasma beta-endorphin levels. Participants were divided into three groups based on participant's pain scale: mild (group I), moderate (group II), and severe (group III). All groups were given WHO 3-step analgesic ladder therapy for 3 days. The participant's pain scale and plasma beta-endorphin level were measured before and after the therapy.
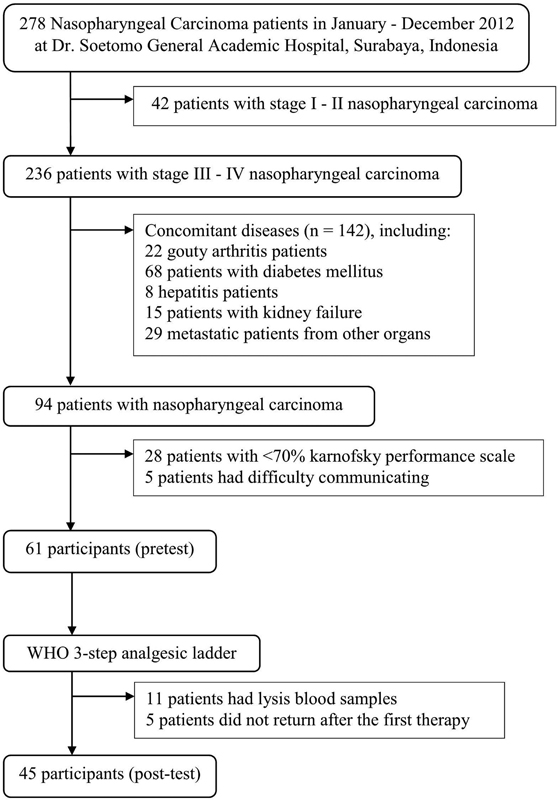
-
Fig. 1 Sampling process in stage III–IV nasopharyngeal carcinoma participants.
The WHO 3-step analgesic ladder therapy given to the participants consisted of three levels: (1) the first level using paracetamol 6 × 500 mg/day and adjuvant amitriptyline 25 to 100 mg/day; (2) the second level using paracetamol 6 × 500 mg/day, codeine with initial dose of 6 × 10 mg/day and increased 10 mg gradually to a maximum dose of 6 × 40 mg/day and adjuvant amitriptyline 25 to 100 mg/day; and (3) the third level using paracetamol 6 × 500 mg/day, morphine with initial dose of 6 × 2.5 mg/day and increased to a maximum of 200 mg/day, and amitriptyline adjuvant 25 to 100 mg/day.
The pain level was measured using VAS. It used a line of 10 cm long, where the left end was the lowest pain value and the right end showed the greatest pain. The participant then decided the value of their pain. The results were categorized into four levels: 0 (no pain); 1 to 3 (mild pain), showing that the pain does not interfere with daily activities and the patient can sleep; 4 to 6 (moderate pain), showing that the pain interferes with daily activities but still can sleep; and 7 to 10 (severe pain), showing that the pain interferes with daily activities and cannot sleep.
Examination of plasma beta-endorphin levels was carried out by taking a ± 10 mL participant cubital venous blood sample. The blood was then stored in a Lavender Vacutainer tube containing EDTA at 2 to 8°C. The tube was put in a cooler box and delivered to the Clinical Pathology Laboratory in the hospital with a cooler box (blood in a cold atmosphere). The blood sample was immediately added with aprotinin 20 μL, before being centrifuged 1,600 g for 15 minutes at 4°C to take blood plasma of 3 cup samples at 0.3 cc plasma. Blood plasma was stored in a cooler at −70°C until all samples were collected. After all samples had been collected, the laboratory staff tested the plasma beta-endorphin level. Beta-endorphins in plasma serum were identified using enzyme-linked immunosorbent assay (MD Biosciences, Zürich, Switzerland). Participant blood samples were taken twice before and after the administration of the WHO 3-step analgesic ladder therapy. Normal plasma endorphin levels are <3 pmol/L (10 pg/mL).
The processed data were tabulated and presented in a tabular form. The statistical tests used paired t-test, Wilcoxon test, and Spearman test. The data obtained were processed using the SPSS 23.0 program (IBM Corp., Armonk, New York, United States). Statistical test results were declared significant if p < 0.05 with 95% confidence interval (CI).
Results
Participant's Characteristics
Most participants aged 51 to 75 years (42.22%), with the youngest and oldest participants' ages being 27 and 72 years, respectively. The average patients' age was 49.24 ± 11.47 years. Most participants were males (71.11%), with a ratio of 2.3: 1 between men and women. Most of the participants were stage-IV NPC patients (68.89%), with most type-III histopathology (84.44%; Table 1).
|
Characteristics |
n (%); N = 45 |
|---|---|
|
Age (y) |
|
|
20–40 |
10 (22.22) |
|
41–50 |
16 (35.56) |
|
51–75 |
19 (42.22) |
|
Sex |
|
|
Male |
32 (71.11) |
|
Female |
13 (28.89) |
|
Education |
|
|
Elementary school |
27 (60.00) |
|
Junior high school |
7 (15.56) |
|
Senior high school |
9 (20.00) |
|
Undergraduate |
2 (4.44) |
|
NPC stage |
|
|
III |
14 (31.11) |
|
IV |
31 (68.89) |
|
Histopathology |
|
|
Type I |
2 (4.44) |
|
Type II |
5 (11.12) |
|
Type III |
38 (84.44) |
Abbreviation: NPC, nasopharyngeal carcinoma.
Most participants were elementary school graduates (60.00%), followed by high school graduates (20.00%). Most elementary school graduates had a moderate pain scale (88.89%), while majority of high school graduates had a moderate pain scale (77.78%). There was no significant correlation between education level and degree of pain (p = 0.408; 95% CI; Table 2).
|
Variables |
Pain (%) |
p-Value |
||
|---|---|---|---|---|
|
Mild (n = 5) |
Moderate (n = 37) |
Severe (n = 3) |
||
|
Education |
0.408 |
|||
|
Elementary school |
2 (7.40) |
24 (88.89) |
1 (3.71) |
|
|
Junior high school |
0 (0.00) |
6 (85.71) |
1 (14.29) |
|
|
Senior high school |
1 (11.11) |
7 (77.78) |
1 (11.11) |
|
|
Undergraduate |
2 (100.00) |
0 (0.00) |
0 (0.00) |
|
|
NPC stage |
0.071 |
|||
|
III |
3 (21.43) |
11 (78.57) |
0 (0.00) |
|
|
IV |
2 (6.45) |
26 (83.87) |
3 (9.68) |
|
Abbreviation: NPC, nasopharyngeal carcinoma.
Nasopharyngeal Carcinoma Stage and Pain Degree
A total of 14 participants had stage-III NPC, of which most participants had moderate pain levels (78.57%). Meanwhile, there were 31 stage-IV NPC participants, and most of them had a moderate pain level (83.87%). There was no significant relationship between NPC stage and degree of pain (p = 0.071; 95% CI; Table 2).
Changes in Degree of Pain during Pre- and Posttherapy
Most participants had a moderate pain level before getting therapy (82.22%). The pain level decreased as most patients had a mild pain level after therapy (66.67%; p < 0.001; 95% CI). Some patients with severe pain scale (6.67%) showed an improvement after therapy, as 13.33% of participants felt no pain. In addition, there were 82.22% of participants who had moderate pain before therapy, and after therapy, the number decreased to 20.00% (Table 3).
|
Pain |
WHO 3-step analgesic ladder therapy |
p-Value |
|
|---|---|---|---|
|
Pre |
Post |
||
|
Pain scale |
0.000a |
||
|
None |
0 (0.00) |
6 (13.33) |
|
|
Mild |
5 (11.11) |
30 (66.67) |
|
|
Moderate |
37 (82.22) |
9 (20.00) |
|
|
Severe |
3 (6.67) |
0 (0.00) |
|
|
Plasma beta-endorphin |
74.89 ± 69.12 |
72.49 ± 75.53 |
0.647 |
The average VAS value before and after therapy was 4.89 ± 1.19 and 2.36, respectively. The minimum and maximum VAS values before therapy were 2.00 and 8.00, respectively. Meanwhile, after therapy, the minimum and maximum VAS values were 0.00 and 6.00, respectively.
Changes in Beta-Endorphin Levels during Pre- and Posttherapy
The average beta-endorphin levels before and after therapy were 74.89 ± 69.12 and 72.49 ± 75.53 pg/mL, respectively (p = 0.647; Table 3). The minimum and maximum values of plasma beta-endorphin levels before therapy were 0.00 and 437.70 pg/mL, respectively. Meanwhile, the minimum and maximum plasma beta-endorphin levels after therapy were 0.80 and 460.40 pg/mL, respectively.
The average plasma beta-endorphin levels in the mild pain group before and after getting therapy were 56.56 ± 35.48 and 37.38 ± 26.02 pg/mL, respectively (p = 0.321). In the moderate pain category, the average plasma beta-endorphin levels before and after therapy were 78.70 ± 74.92 and 80.12 ± 80.90 pg/mL, respectively (p = 0.805). On the other hand, the average plasma beta-endorphin levels in the severe pain category before and after therapy were 58.47 ± 6.27 and 36.83 ± 18.05 pg/mL, respectively (p = 0.235).
Pain Degree and Plasma Beta-Endorphin Level
The average plasma beta-endorphin levels before therapy were 56.56 ± 35.48 pg/mL (mild pain group), 78.70 ± 74.92 pg/mL (moderate pain group), and 58.47 ± 6.27 pg/mL (severe pain group; p = 0.717). Whereas after therapy, the average plasma beta-endorphin levels were 44.03 ± 28.40 pg/mL (mild pain group), 75.50 ± 85.01 pg/mL (moderate pain group), and 81.42 ± 62.84 pg/mL (severe pain group; p = 0.213). There was a difference in the value of average plasma beta-endorphin levels before and after therapy in the mild pain group of −19.20 ± 37.72 pg/mL, in the moderate pain group of −4.76 ± 35.30 pg/mL, and in the severe pain group of −21.67 ± 6.27 pg/mL (p = 0.717; Table 4).
|
Beta endorphin |
Pain (mean ± SD) |
p-Value |
||
|---|---|---|---|---|
|
Mild (n = 5) |
Moderate (n = 37) |
Severe (n = 3) |
||
|
Pre |
56.56 ± 35.48 |
78.70 ± 74.92 |
58.47 ± 6.27 |
0.717 |
|
Post |
44.03 ± 28.40 |
75.50 ± 85.01 |
81.42 ± 62.84 |
0.213 |
|
Pre-Post |
−19.20 ± 37.72 |
−4.76 ± 35.30 |
−21.67 ± 21.94 |
0.732 |
Abbreviation: SD, standard deviation.
Discussion
This study found a significant difference in the degree of pain before and after therapy. The use of WHO 3-step analgesic ladder therapy is a safe and effective drug proven to be able to reduce the pain scale of NPC patients according to the level of pain experienced by the patient.15 Some literatures mentioned that the degree of pain in NPC patients after analgesic therapy decreased. Changes in pain scale indicate that the more severe the degree of pain, the higher doses of analgesics and stronger types of opioid analgesics the patient will need.8,16,17 The WHO since 1986 has issued a guideline on how to deal with pain in cancer patients. It mentioned that giving therapy to cancer patients who experience pain has a success rate between 70 and 90%. In order to achieve the success rate, the patient must follow the recommended method of giving oral therapy and on-time analgesic therapy. In accordance with the WHO 3-step analgesic ladder principle, the dose given is adjusted individually because there is no standard dose for opioid administration, and the medical personnel should give a detailed explanation to patients about the treatment to be undertaken.5,6,18
This study found no significant differences in plasma beta-endorphin levels before and after the therapy in participants with advanced NPC. In addition, plasma endorphin levels before and after therapy in each degree of pain did not show a significant difference either. There is still a conflict about the action mechanism of beta-endorphins to relieve pain, the process, and the psychological influence on pain. The limitations of ethical research directly on humans are one obstacle in knowing better the effects of beta-endorphins in the human brain. Therefore, there are only indirect research studies to measure plasma endorphin levels in humans.19 In addition to acting on receptors in the brain in the analgesic system, beta-endorphins are released through portal blood vessels into the general circulation. However, beta-endorphins in the circulation will quickly undergo metabolism. Giving exogenous beta-endorphin injections in various places in the brain, including the arctic nucleus or in the cerebrospinal fluid, will provide a stronger analgesic effect compared to morphine. However, intravenous administration of beta-endorphins by infusion does not have an analgesic effect.20,21 Beta-endorphins in peripheral blood are not related to pain but are a reflection of perception and stress reactions to pain.22
This study found no significant relationship between changes in the degree of pain and changes in plasma beta-endorphin levels before and after therapy. Previous research studies also stated that analgesia was associated with a significant increase in plasma beta-endorphin levels before therapy and after loss of pain. Plasma beta-endorphin levels are inversely proportional to the VAS scale.19,23 Plasma beta-endorphins will affect the central nervous system because of their ability to penetrate the blood–brain barrier, and the release of beta-endorphins in the brain will affect plasma beta-endorphin levels through portal blood vessels.10,11
This study showed the benefits of treating NPC pain using the WHO principle, where the success rate was above 70%. However, this study did not obtain expected results from the measurement of plasma beta-endorphins, as it was likely influenced by the long evaluation time that caused a decreased plasma level.
The limitations of this study included the small sample size, even though we had collected a lot of participants for 1 year. This study was not a randomized control trial, so further studies are expected to conduct research using the randomized design in order to get better results.
Conclusion
The degree of pain in advanced NPC patients decreased after therapy. Plasma beta-endorphin levels of NPC patients before and after therapy do not have a significant difference. There is no significant relationship between decreasing degree of pain and an increase in plasma beta-endorphin levels of NPC patients before and after therapy.
Acknowledgement
We would like to thank Fis Citra Ariyanto for being our manuscript editor.
Conflict of Interest
None declared.
References
- Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer. 2012;31(04):185-196.
- [Google Scholar]
- Pain in head and neck cancer: prevalence and possible predictive factors. J BUON. 2014;19(03):592-597.
- [Google Scholar]
- Characteristics of chronic pain among head and neck cancer patients treated with radiation therapy: a retrospective study. Pain Res Manag. 2019;2019:9675654.
- [Google Scholar]
- Standardized nursing and therapeutic effect of oxycontin on oral mucosal pain in nasopharyngeal carcinoma patients. J Cancer Res Ther. 2018;14(07):1594-1599.
- [Google Scholar]
- Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res. 2016;9:515-534.
- [Google Scholar]
- Cancer pain management-current status. J Anaesthesiol Clin Pharmacol. 2011;27(02):162-168.
- [Google Scholar]
- Efficacy of controlled-release oxycodone for reducing pain due to oral mucositis in nasopharyngeal carcinoma patients treated with concurrent chemoradiotherapy: a prospective clinical trial. Support Care Cancer. 2019;27(10):3759-3767.
- [Google Scholar]
- Pharmacological and other interventions for head and neck cancer pain: a systematic review. J Oral Maxillofac Res. 2013;3(04):e1.
- [Google Scholar]
- Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: position paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC) Allergo J Int. 2017;26(01):16-24.
- [Google Scholar]
- Regulation of cancer progression by β-endorphin neuron. Cancer Res. 2012;72(04):836-840.
- [Google Scholar]
- The effects of beta-endorphin: state change modification. Fluids Barriers CNS. 2015;12:3.
- [Google Scholar]
- What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function? Eur J Pain. 2012;16(03):370-380.
- [Google Scholar]
- Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730-6738.
- [Google Scholar]
- Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(03):233-244.
- [Google Scholar]
- Transdermal fentanyl for pain due to chemoradiotherapy-induced oral mucositis in nasopharyngeal cancer patients: evaluating efficacy, safety, and improvement in quality of life. Drug Des Devel Ther. 2014;8:497-503.
- [Google Scholar]
- Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand. 2002;46(07):759-770.
- [Google Scholar]
- The burden of chronic pain after major head and neck tumor therapy. Saudi J Anaesth. 2017;11(01):S71-S79.
- [Google Scholar]
- Treatment of pain in cancer: towards personalised medicine. Cancers (Basel). 2018;10(12):502.
- [Google Scholar]
- Plasma Beta-endorphin levels before and after relief of cancer pain. Pain Physician. 2004;7(01):67-70.
- [Google Scholar]
- Nociceptive stimulus induces release of endogenous β-endorphin in the rat brain. Neuroscience. 1998;85(03):659-662.
- [Google Scholar]
- Reducing adsorption to improve recovery and in vivo detection of neuropeptides by microdialysis with LC-MS. Anal Chem. 2015;87(19):9802-9809.
- [Google Scholar]
- Effect of naltrexone on neuropathic pain in mice locally transfected with the mutant μ-opioid receptor gene in spinal cord. Br J Pharmacol. 2015;172(02):630-641.
- [Google Scholar]
- Combined analysis of circulating β-endorphin with gene polymorphisms in OPRM1, CACNAD2 and ABCB1 reveals correlation with pain, opioid sensitivity and opioid-related side effects. Mol Brain. 2013;6:8.
- [Google Scholar]







